The effect of risedronate on hip structural geometry in chemotherapy-induced postmenopausal women with or without use of aromatase inhibitors: a 2-year trial
- PMID: 19853678
- PMCID: PMC2857766
- DOI: 10.1016/j.bone.2009.10.019
The effect of risedronate on hip structural geometry in chemotherapy-induced postmenopausal women with or without use of aromatase inhibitors: a 2-year trial
Abstract
Introduction: Osteoporosis is a major health problem for postmenopausal women. Adjuvant hormonal therapy with aromatase inhibitors (AIs) in postmenopausal breast cancer patients further worsens bone loss. Bisphosphonates are able to prevent AI-induced bone loss, but limited data exists on their effect on bone structure. Our objectives were to (1) examine the impact of AIs and no-AIs on hip structural geometry (HSA) of chemotherapy-induced postmenopausal women, and (2) determine if oral bisphosphonates could affect these changes.
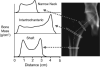
Methods: This is a sub-analysis of a 2-year double-blind randomized trial of 67 women with nonmetastatic breast cancer, newly postmenopausal following chemotherapy (up to 8 years), who were randomized to risedronate, 35 mg once weekly (RIS) and placebo (PBO). Many women changed their cancer therapy from a no-AI to an AI during the trial. Outcomes were changes in Beck's HSA-derived BMD and structural parameters.
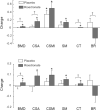
Results: Eighteen women did not receive adjuvant hormone therapy, while 41 women received other therapy and 8 received AIs at baseline distributed similarly between RIS and PBO. Women on AIs and PBO were found to have the lowest BMD and indices. RIS improved BMD and several HSA indices at the intertrochanteric site in women regardless of their hormonal therapy, but most improvement was observed in women who were not on AIs (all p< or =0.05 except buckling ratio). Changes at the narrow neck and femoral shaft were similar.
Conclusion: The use of AIs appears to lead to lower HSA-derived BMD and hip structural indices as compared to women on no or non-AI therapy in chemotherapy-induced postmenopausal breast cancer patients. Preventive therapy with once weekly oral risedronate maintains structural, skeletal integrity independently of the use of or type of adjuvant therapy.
Figures


References
-
- Brufsky AM. Bone health issues in women with early-stage breast cancer receiving aromatase inhibitors. Curr Oncol Rep. 2008;10:18–26. - PubMed
-
- Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev. 2008;34(Suppl 1):S3–18. - PubMed
-
- Coleman RE, Body JJ, Gralow JR, Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treat Rev. 2008;34(Suppl 1):S31–42. - PubMed
-
- Hadji P, Body JJ, Aapro MS, Brufsky A, Coleman RE, Guise T, Lipton A, Tubiana-Hulin M. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann Oncol. 2008;19:1407–16. - PubMed
-
- Coleman RE, Body J-J, Gralow JR, Lipton A. Bone loss in patients with breast cancer receiving aromatase inhibitors and associated treatment strategies. Cancer Treatment Reviews. 2008;34:S31–S42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

