Nucleotide oligomerization domain-2 interacts with 2'-5'-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells
- PMID: 19853919
- PMCID: PMC2787966
- DOI: 10.1016/j.molimm.2009.09.025
Nucleotide oligomerization domain-2 interacts with 2'-5'-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells
Abstract
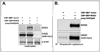
Nucleotide-binding and oligomerization domain-2 (NOD2) is an intracellular protein involved in innate immunity and linked to chronic inflammatory diseases in humans. Further characterization of the full spectrum of proteins capable of binding to NOD2 may provide new insights into its normal functioning as well as the mechanisms by which mutated forms cause disease. Using a proteomics approach to study human THP-1 cells, we have identified 2'-5'-oligoadenylate synthetase type 2 (OAS2), a dsRNA binding protein involved in the pathway that activates RNase-L, as a new binding partner for NOD2. The interaction was confirmed using over-expression of OAS2 and NOD2 in HEK cells. Further confirmation was obtained by detecting NOD2 in immunoprecipitates of endogenous OAS2 in THP-1 cells. Finally, over-expression of NOD2 in THP-1 cells led to enhanced RNase-L activity in cells treated with poly(I:C), a mimic of double-stranded RNA virus infection. These data indicate connectivity in pathways involved in innate immunity to bacteria and viruses and suggest a regulatory role whereby NOD2 enhances the function of RNase-L.
Figures



References
-
- Andersen JB, Strandbygard DJ, Hartmann R, Justesen J. Interaction between the 2'-5' oligoadenylate synthetase-like protein p59 OASL and the transcriptional repressor methyl CpG-binding protein 1. Eur J Biochem. 2004;271:628–636. - PubMed
-
- Barnich N, Hisamatsu T, Aguirre JE, Xavier R, Reinecker HC, Podolsky DK. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J Biol Chem. 2005b;280:19021–19026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

