Hepatitis C virus hijacks host lipid metabolism
- PMID: 19854061
- PMCID: PMC2818172
- DOI: 10.1016/j.tem.2009.07.005
Hepatitis C virus hijacks host lipid metabolism
Abstract
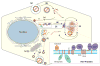
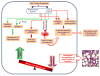
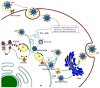
Hepatitis C virus (HCV) enhances its replication by modulating host cell lipid metabolism. HCV circulates in the blood in association with lipoproteins. HCV infection is associated with enhanced lipogenesis, reduced secretion, and beta-oxidation of lipids. HCV-induced imbalance in lipid homeostasis leads to steatosis. Many lipids are crucial for the virus life cycle, and inhibitors of cholesterol/fatty acid biosynthetic pathways inhibit virus replication, maturation and secretion. HCV negatively modulates the synthesis and secretion of very low-density lipoproteins (VLDL). Components involved in VLDL assembly are also required for HCV morphogenesis/secretion, suggesting that HCV co-opts the VLDL secretory pathway for its own secretion. This review highlights HCV-altered lipid metabolic events that aid the virus life cycle and ultimately promote liver disease.
Figures
References
-
- Shepard CW, et al. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. - PubMed
-
- Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29(Suppl 2):26–37. - PubMed
-
- Dubuisson J, et al. Interaction of hepatitis C virus proteins with host cell membranes and lipids. Trends Cell Biol. 2002;12:517–523. - PubMed
-
- Mannova P, et al. Modification of host lipid raft proteome upon hepatitis C virus replication. Mol Cell Proteomics. 2006;5:2319–2325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

