Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial
- PMID: 19854499
- PMCID: PMC4492302
- DOI: 10.1016/S0140-6736(09)61836-5
Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial
Erratum in
- Lancet. 2010 Jan 2;375(9708):30
Abstract
Background: Gene therapy has the potential to reverse disease or prevent further deterioration of vision in patients with incurable inherited retinal degeneration. We therefore did a phase 1 trial to assess the effect of gene therapy on retinal and visual function in children and adults with Leber's congenital amaurosis.
Methods: We assessed the retinal and visual function in 12 patients (aged 8-44 years) with RPE65-associated Leber's congenital amaurosis given one subretinal injection of adeno-associated virus (AAV) containing a gene encoding a protein needed for the isomerohydrolase activity of the retinal pigment epithelium (AAV2-hRPE65v2) in the worst eye at low (1.5 x 10(10) vector genomes), medium (4.8 x 10(10) vector genomes), or high dose (1.5 x 10(11) vector genomes) for up to 2 years.
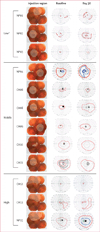
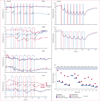
Findings: AAV2-hRPE65v2 was well tolerated and all patients showed sustained improvement in subjective and objective measurements of vision (ie, dark adaptometry, pupillometry, electroretinography, nystagmus, and ambulatory behaviour). Patients had at least a 2 log unit increase in pupillary light responses, and an 8-year-old child had nearly the same level of light sensitivity as that in age-matched normal-sighted individuals. The greatest improvement was noted in children, all of whom gained ambulatory vision. The study is registered with ClinicalTrials.gov, number NCT00516477.
Interpretation: The safety, extent, and stability of improvement in vision in all patients support the use of AAV-mediated gene therapy for treatment of inherited retinal diseases, with early intervention resulting in the best potential gain.
Funding: Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia, Foundation Fighting Blindness, Telethon, Research to Prevent Blindness, F M Kirby Foundation, Mackall Foundation Trust, Regione Campania Convenzione, European Union, Associazione Italiana Amaurosi Congenita di Leber, Fund for Scientific Research, Fund for Research in Ophthalmology, and National Center for Research Resources.
Conflict of interest statement
The other authors declare that they have no conflicts of interest.
Figures

Comment in
-
Promises and challenges of genetic therapy for blindness.Lancet. 2009 Nov 7;374(9701):1569-70. doi: 10.1016/S0140-6736(09)61869-9. Epub 2009 Oct 23. Lancet. 2009. PMID: 19854500 No abstract available.
References
-
- Aleman TS, Jacobson SG, Chico JD, et al. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:1259–1271. - PubMed
-
- Lorenz B, Gyurus P, Preising M, et al. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci. 2000;41:2735–2742. - PubMed
-
- Simonelli F, Ziviello C, Testa F, et al. Clinical and molecular genetics of Leber’s congenital amaurosis: A multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–4290. - PubMed
-
- Perrault I, Rozet JM, Gerber S, et al. Leber congenital amaurosis. Mol Genet Metab. 1999 Oct;68:200–208. - PubMed
-
- den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retinal Eye Res. 2008;27:391–419. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

