Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter
- PMID: 19854866
- PMCID: PMC2795705
- DOI: 10.1210/en.2009-0881
Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter
Abstract
CRH is widely expressed in the brain and is of broad functional relevance to a number of physiological processes, including stress response, parturition, immune response, and ingestive behavior. To delineate further the organization of the central CRH network, we generated mice expressing green fluorescent protein (GFP) under the control of the CRH promoter, using bacterial artificial chromosome technology. Here we validate CRH-GFP transgene expression within specific brain regions and confirm the distribution of central GFP-producing cells to faithfully recapitulate that of CRH-expressing cells. Furthermore, we confirm the functional integrity of a population of GFP-producing cells by demonstrating their opposite responsiveness to nutritional status. We anticipate that this transgenic model will lend itself as a highly tractable tool for the investigation of CRH expression and function in discrete brain regions.
Figures

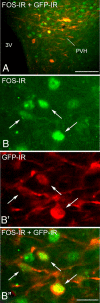
References
-
- Bamberger CM, Bamberger AM 2000 The peripheral CRH/urocortin system. Ann NY Acad Sci 917:290–296 - PubMed
-
- Keegan CE, Herman JP, Karolyi IJ, O'Shea KS, Camper SA, Seasholtz AF 1994 Differential expression of corticotropin-releasing hormone in developing mouse embryos and adult brain. Endocrinology 134:2547–2555 - PubMed
-
- Merchenthaler I, Vigh S, Petrusz P, Schally AV 1982 Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat 165:385–396 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

