Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori
- PMID: 19854893
- PMCID: PMC2798266
- DOI: 10.1128/JB.00848-09
Cytoplasmic histidine kinase (HP0244)-regulated assembly of urease with UreI, a channel for urea and its metabolites, CO2, NH3, and NH4(+), is necessary for acid survival of Helicobacter pylori
Abstract
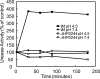
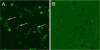
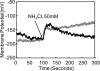
Helicobacter pylori colonizes the normal human stomach by maintaining both periplasmic and cytoplasmic pH close to neutral in the presence of gastric acidity. Urease activity, urea flux through the pH-gated urea channel, UreI, and periplasmic alpha-carbonic anhydrase are essential for colonization. Exposure to pH 4.5 for up to 180 min activates total bacterial urease threefold. Within 30 min at pH 4.5, the urease structural subunits, UreA and UreB, and the Ni(2+) insertion protein, UreE, are recruited to UreI at the inner membrane. Formation of this complex and urease activation depend on expression of the cytoplasmic sensor histidine kinase, HP0244. Its deletion abolishes urease activation and assembly, impairs cytoplasmic and periplasmic pH homeostasis, and depolarizes the cells, with an approximately 7-log loss of survival at pH 2.5, even in 10 mM urea. Associated with this assembly, UreI is able to transport NH(3), NH(4)(+), and CO(2), as shown by changes in cytoplasmic pH following exposure to NH(4)Cl or CO(2). To be able to colonize cells in the presence of the highly variable pH of the stomach, the organism expresses two pH-sensor histidine kinases, one, HP0165, responding to a moderate fall in periplasmic pH and the other, HP0244, responding to cytoplasmic acidification at a more acidic medium pH. Assembly of a pH-regulatory complex of active urease with UreI provides an advantage for periplasmic buffering.
Figures



References
-
- Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. - PubMed
-
- Bury-Mone, S., G. L. Mendz, G. E. Ball, M. Thibonnier, K. Stingl, C. Ecobichon, P. Ave, M. Huerre, A. Labigne, J. M. Thiberge, and H. De Reuse. 2008. Roles of alpha and beta carbonic anhydrases of Helicobacter pylori in the urease-dependent response to acidity and in colonization of the murine gastric mucosa. Infect. Immun. 76:497-509. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

