Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents
- PMID: 19854938
- PMCID: PMC2800216
- DOI: 10.1093/nar/gkp841
Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents
Abstract
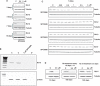
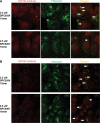
For the past 15-20 years, the intracellular delivery and silencing activity of oligodeoxynucleotides have been essentially completely dependent on the use of a delivery technology (e.g. lipofection). We have developed a method (called 'gymnosis') that does not require the use of any transfection reagent or any additives to serum whatsoever, but rather takes advantage of the normal growth properties of cells in tissue culture in order to promote productive oligonucleotide uptake. This robust method permits the sequence-specific silencing of multiple targets in a large number of cell types in tissue culture, both at the protein and mRNA level, at concentrations in the low micromolar range. Optimum results were obtained with locked nucleic acid (LNA) phosphorothioate gap-mers. By appropriate manipulation of oligonucleotide dosing, this silencing can be continuously maintained with little or no toxicity for >240 days. High levels of oligonucleotide in the cell nucleus are not a requirement for gene silencing, contrary to long accepted dogma. In addition, gymnotic delivery can efficiently deliver oligonucleotides to suspension cells that are known to be very difficult to transfect. Finally, the pattern of gene silencing of in vitro gymnotically delivered oligonucleotides correlates particularly well with in vivo silencing. The establishment of this link is of particular significance to those in the academic research and drug discovery and development communities.
Figures



References
-
- Moulds C, Lewis J, Froehler B, Grant D, Huang T, Milligan J, Matteucci M, Wagner R. Site and mechanism of antisense inhibition of C-5 propyne oligonucleotides. Biochemistry. 1995;34:5044–5053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

