Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells
- PMID: 19855003
- PMCID: PMC2776415
- DOI: 10.1073/pnas.0904343106
Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells
Abstract
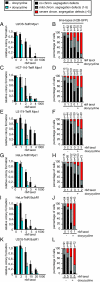
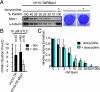
The mitotic checkpoint has evolved to prevent chromosome mis-segregations by delaying mitosis when unattached chromosomes are present. Inducing severe chromosome segregation errors by ablating the mitotic checkpoint causes cell death. Here we have analyzed the consequences of gradual increases in chromosome segregation errors on the viability of tumor cells and normal human fibroblasts. Partial reduction of essential mitotic checkpoint components in four tumor cell lines caused mild chromosome mis-segregations, but no lethality. These cells were, however, remarkably more sensitive to low doses of taxol, which enhanced the amount and severity of chromosome segregation errors. Sensitization to taxol was achieved by reducing levels of Mps1 or BubR1, proteins having dual roles in checkpoint activation and chromosome alignment, but not by reducing Mad2, functioning solely in the mitotic checkpoint. Moreover, we find that untransformed human fibroblasts with reduced Mps1 levels could not be sensitized to sublethal doses of taxol. Thus, targeting the mitotic checkpoint and chromosome alignment simultaneously may selectively kill tumor cells by enhancing chromosome mis-segregations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cohen J. Sorting out chromosome errors. Science. 2002;296:2164–2166. - PubMed
-
- Heim Sa, Mitelman F. Cancer Cytogenetics. New York: Wiley Liss Inc.; 1995.
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. - PubMed
-
- Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18:658–667. - PubMed
-
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

