The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells
- PMID: 19855016
- PMCID: PMC2766341
- DOI: 10.1242/dev.037317
The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells
Abstract
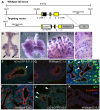
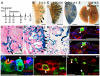
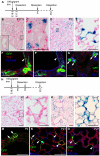
The conducting airways (bronchi and bronchioles) and peripheral gas exchange (alveolar) regions of the mammalian lung are generated by a process of branching morphogenesis. Evidence suggests that during embryonic development, the undifferentiated epithelial progenitors are located at the distal tips of the branching epithelium. To test this hypothesis, we used an Id2-CreER(T2) knock-in mouse strain to lineage trace the distal epithelial tip cells during either the pseudoglandular or canalicular phases of development. During the pseudoglandular stage, the tip cells both self-renew and contribute descendents to all epithelial cell lineages, including neuroendocrine cells. In addition, individual Id2(+) tip cells can self-renew and contribute descendents to both the bronchiolar and alveolar compartments. By contrast, during the later canalicular stage, the distal epithelial tip cells only contribute descendents to the alveoli. Taken together, this evidence supports a model in which the distal tip of the developing lung contains a multipotent epithelial population, the fate of which changes during development.
Figures

References
-
- Cardoso, W. V. and Lu, J. (2006). Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611-1624. - PubMed
-
- Eblaghie, M. C., Reedy, M., Oliver, T., Mishina, Y. and Hogan, B. L. (2006). Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 291, 67-82. - PubMed
-
- Farley, F. W., Soriano, P., Steffen, L. S. and Dymecki, S. M. (2000). Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28, 106-110. - PubMed
-
- Hayashi, S. and McMahon, A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305-318. - PubMed
-
- Hong, K. U., Reynolds, S. D., Giangreco, A., Hurley, C. M. and Stripp, B. R. (2001). Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am. J. Respir. Cell Mol. Biol. 24, 671-681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

