Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus
- PMID: 19855383
- PMCID: PMC3415301
- DOI: 10.1038/nmat2558
Nanofibrous biologic laminates replicate the form and function of the annulus fibrosus
Abstract
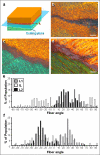
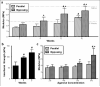
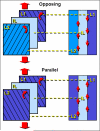
Successful engineering of load-bearing tissues requires recapitulation of their complex mechanical functions. Given the intimate relationship between function and form, biomimetic materials that replicate anatomic form are of great interest for tissue engineering applications. However, for complex tissues such as the annulus fibrosus, scaffolds have failed to capture their multi-scale structural hierarchy. Consequently, engineered tissues have yet to reach functional equivalence with their native counterparts. Here, we present a novel strategy for annulus fibrosus tissue engineering that replicates this hierarchy with anisotropic nanofibrous laminates seeded with mesenchymal stem cells. These scaffolds directed the deposition of an organized, collagen-rich extracellular matrix that mimicked the angle-ply, multi-lamellar architecture and achieved mechanical parity with native tissue after 10 weeks of in vitro culture. Furthermore, we identified a novel role for inter-lamellar shearing in reinforcing the tensile response of biologic laminates, a mechanism that has not previously been considered for these tissues.
Figures


Comment in
-
Tissue engineering: Function follows form.Nat Mater. 2009 Dec;8(12):923-4. doi: 10.1038/nmat2577. Nat Mater. 2009. PMID: 19935690 Free PMC article. No abstract available.
References
-
- Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75–88. - PubMed
-
- Elliott DM, Setton LA. Anisotropic and inhomogeneous tensile behavior of the human anulus fibrosus: experimental measurement and material model predictions. J Biomech Eng. 2001;123:256–263. - PubMed
-
- Hukins DW. Tissue engineering: a live disc. Nat Mater. 2005;4:881–2. - PubMed
-
- Place ES, George JH, Williams CK, Stevens MM. Synthetic polymer scaffolds for tissue engineering. Chem Soc Rev. 2009;38:1139–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

