Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse
- PMID: 19855387
- PMCID: PMC2837911
- DOI: 10.1038/ncb1977
Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse
Abstract
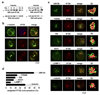
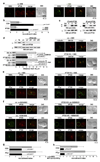
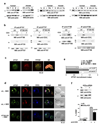
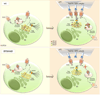
Most eukaryotic cells have a primary cilium which functions as a sensory organelle. Cilia are assembled by intraflagellar transport (IFT), a process mediated by multimeric IFT particles and molecular motors. Here we show that lymphoid and myeloid cells, which lack primary cilia, express IFT proteins. IFT20, an IFT component essential for ciliary assembly, was found to colocalize with both the microtubule organizing centre (MTOC) and Golgi and post-Golgi compartments in T-lymphocytes. In antigen-specific conjugates, IFT20 translocated to the immune synapse. IFT20 knockdown resulted in impaired T-cell receptor/CD3 (TCR/CD3) clustering and signalling at the immune synapse, due to defective polarized recycling. Moreover, IFT20 was required for the inducible assembly of a complex with other IFT components (IFT57 and IFT88) and the TCR. The results identify IFT20 as a new regulator of immune synapse assembly in T cells and provide the first evidence to implicate IFT in membrane trafficking in cells lacking primary cilia, thereby introducing a new perspective on IFT function beyond its role in ciliogenesis.
Figures

References
-
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439. - PubMed
-
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813. - PubMed
-
- Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

