Extracellular histones are major mediators of death in sepsis
- PMID: 19855397
- PMCID: PMC2783754
- DOI: 10.1038/nm.2053
Extracellular histones are major mediators of death in sepsis
Abstract
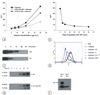
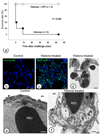
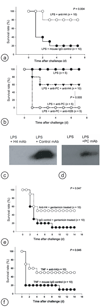
Hyperinflammatory responses can lead to a variety of diseases, including sepsis. We now report that extracellular histones released in response to inflammatory challenge contribute to endothelial dysfunction, organ failure and death during sepsis. They can be targeted pharmacologically by antibody to histone or by activated protein C (APC). Antibody to histone reduced the mortality of mice in lipopolysaccharide (LPS), tumor necrosis factor (TNF) or cecal ligation and puncture models of sepsis. Extracellular histones are cytotoxic toward endothelium in vitro and are lethal in mice. In vivo, histone administration resulted in neutrophil margination, vacuolated endothelium, intra-alveolar hemorrhage and macro- and microvascular thrombosis. We detected histone in the circulation of baboons challenged with Escherichia coli, and the increase in histone levels was accompanied by the onset of renal dysfunction. APC cleaves histones and reduces their cytotoxicity. Co-infusion of APC with E. coli in baboons or histones in mice prevented lethality. Blockade of protein C activation exacerbated sublethal LPS challenge into lethality, which was reversed by treatment with antibody to histone. We conclude that extracellular histones are potential molecular targets for therapeutics for sepsis and other inflammatory diseases.
Figures

Comment in
-
Sepsis: the dark side of histones.Nat Med. 2009 Nov;15(11):1245-6. doi: 10.1038/nm1109-1245. Nat Med. 2009. PMID: 19893552 No abstract available.
References
-
- Wang H, et al. HMG–1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. - PubMed
-
- Bernard GR, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. - PubMed
-
- Russell JA. Management of sepsis. N. Engl. J. Med. 2006;355:1699–1713. - PubMed
-
- Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

