Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines
- PMID: 19855398
- PMCID: PMC3616497
- DOI: 10.1038/nm.2036
Salmonella disrupts lymph node architecture by TLR4-mediated suppression of homeostatic chemokines
Abstract
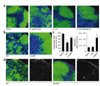
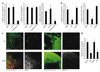
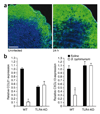
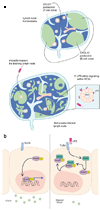
We report that infection of draining lymph nodes (DLNs) by Salmonella typhimurium results in the specific downregulation of the homeostatic chemokines CCL21 and CXCL13, which are essential for normal DLN organization and function. Our data reveal that the mechanism of this suppression is dependent on S. typhimurium LPS (sLPS). The decrease in CCL21 expression involves interaction between sLPS and CCL21-producing cells within DLNs, triggering a distinct Toll-like receptor 4 (TLR4)-mediated host signaling response. In this response, suppressor of cytokine signaling-3 (Socs3) is upregulated, which negatively regulates mothers against decapentaplegic homolog-3 (Smad3)-initiated production of CCL21. Disruption of lymph node architecture and cellular trafficking enhances S. typhimurium virulence and could represent a mechanism of immune suppression used by pathogens that primarily target lymphoid tissue.
Figures


References
-
- Gunn MD, et al. A B cell–homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. - PubMed
-
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 2003;3:867–878. - PubMed
-
- Cyster JG, et al. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 2000;176:181–193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

