Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency
- PMID: 19855399
- PMCID: PMC2783573
- DOI: 10.1038/nm.2051
Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency
Abstract
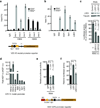
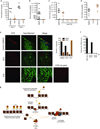
Reversible methylation of histone tails serves as either a positive signal recognized by transcriptional assemblies or a negative signal that result in repression. Invading viral pathogens that depend upon the host cell's transcriptional apparatus are also subject to the regulatory impact of chromatin assembly and modifications. Here we show that infection by the alpha-herpesviruses, herpes simplex virus (HSV) and varicella zoster virus (VZV), results in the rapid accumulation of chromatin bearing repressive histone H3 Lys9 methylation. To enable expression of viral immediate early (IE) genes, both viruses use the cellular transcriptional coactivator host cell factor-1 (HCF-1) to recruit the lysine-specific demethylase-1 (LSD1) to the viral immediate early promoters. Depletion of LSD1 or inhibition of its activity with monoamine oxidase inhibitors (MAOIs) results in the accumulation of repressive chromatin and a block to viral gene expression. As HCF-1 is a component of the Set1 and MLL1 histone H3 Lys4 methyltransferase complexes, it thus coordinates modulation of repressive H3 Lys9 methylation levels with addition of activating H3 Lys4 trimethylation marks. Strikingly, MAOIs also block the reactivation of HSV from latency in sensory neurons, indicating that the HCF-1 complex is a crucial component of the reactivation mechanism. The results support pharmaceutical control of histone modifying enzymes as a strategy for controlling herpesvirus infections.
Figures


References
-
- Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–318. - PubMed
-
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. - PubMed
-
- Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25:1–14. - PubMed
-
- Kouzarides T, Berger SL. Chromatin Modifications and Their Mechanism of Action. In: Allis CD, Jenuwein T, Reinberg D, editors. Epigenetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 191–209.
-
- Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

