Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation
- PMID: 19855428
- PMCID: PMC2795787
- DOI: 10.1038/onc.2009.270
Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation
Abstract
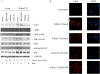
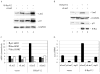
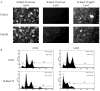
The Lats2 tumor suppressor protein has been implicated earlier in promoting p53 activation in response to mitotic apparatus stress, by preventing Mdm2-driven p53 degradation. We now report that Lats2 also has a role in an ATR-Chk1-mediated stress check point in response to oncogenic H-Ras. Activated mutant H-Ras triggers the translocation of Lats2 from centrosomes into the nucleus, coupled with an increase in Lats2 protein levels. This leads to the induction of p53 activity, upregulation of proapoptotic genes, downregulation of antiapoptotic genes and eventually apoptotic cell death. Many of the cells that survive apoptosis undergo senescence. However, a fraction of the cells escape this checkpoint mechanism, despite maintaining a high mutant H-Ras expression. These escapers display increased genome instability, as evidenced by a substantial fraction of cells with micronuclei and cells with polyploid genomes. Interestingly, such cells show markedly reduced levels of Lats2, in conjunction with enhanced hypermethylation of the Lats2 gene promoter. Our findings suggest that Lats2 might have an important role in quenching H-Ras-induced transformation, whereas silencing of Lats2 expression might serve as a mechanism to enable tumor progression.
Figures



References
-
- Abulaiti A, Fikaris AJ, Tsygankova OM, Meinkoth JL. Ras induces chromosome instability and abrogation of the DNA damage response. Cancer Res. 2006;66(21):10505–10512. - PubMed
-
- Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26(56):7773–7779. - PubMed
-
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–637. - PubMed
-
- Bean GR, Bryson AD, Pilie PG, Goldenberg V, Baker JC, Jr, Ibarra C, et al. Morphologically normal-appearing mammary epithelial cells obtained from high-risk women exhibit methylation silencing of INK4a/ARF. Clin Cancer Res. 2007;13(22 Pt 1):6834–6841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

