A computational framework for ultrastructural mapping of neural circuitry
- PMID: 19855814
- PMCID: PMC2661966
- DOI: 10.1371/journal.pbio.1000074
A computational framework for ultrastructural mapping of neural circuitry
Abstract
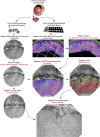
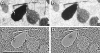
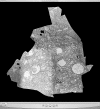
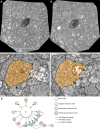
Circuitry mapping of metazoan neural systems is difficult because canonical neural regions (regions containing one or more copies of all components) are large, regional borders are uncertain, neuronal diversity is high, and potential network topologies so numerous that only anatomical ground truth can resolve them. Complete mapping of a specific network requires synaptic resolution, canonical region coverage, and robust neuronal classification. Though transmission electron microscopy (TEM) remains the optimal tool for network mapping, the process of building large serial section TEM (ssTEM) image volumes is rendered difficult by the need to precisely mosaic distorted image tiles and register distorted mosaics. Moreover, most molecular neuronal class markers are poorly compatible with optimal TEM imaging. Our objective was to build a complete framework for ultrastructural circuitry mapping. This framework combines strong TEM-compliant small molecule profiling with automated image tile mosaicking, automated slice-to-slice image registration, and gigabyte-scale image browsing for volume annotation. Specifically we show how ultrathin molecular profiling datasets and their resultant classification maps can be embedded into ssTEM datasets and how scripted acquisition tools (SerialEM), mosaicking and registration (ir-tools), and large slice viewers (MosaicBuilder, Viking) can be used to manage terabyte-scale volumes. These methods enable large-scale connectivity analyses of new and legacy data. In well-posed tasks (e.g., complete network mapping in retina), terabyte-scale image volumes that previously would require decades of assembly can now be completed in months. Perhaps more importantly, the fusion of molecular profiling, image acquisition by SerialEM, ir-tools volume assembly, and data viewers/annotators also allow ssTEM to be used as a prospective tool for discovery in nonneural systems and a practical screening methodology for neurogenetics. Finally, this framework provides a mechanism for parallelization of ssTEM imaging, volume assembly, and data analysis across an international user base, enhancing the productivity of a large cohort of electron microscopists.
Conflict of interest statement
Competing interests. Robert E. Marc is a principal of Signature Immunologics. All other authors declare no other competing interests.
Figures










References
-
- Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. doi: 10.1371/journal.pcbi.0010042. - DOI - PMC - PubMed
-
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. - PubMed
-
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol. 2004;472:73–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

