A comparative study of Drosophila and human A-type lamins
- PMID: 19855837
- PMCID: PMC2762312
- DOI: 10.1371/journal.pone.0007564
A comparative study of Drosophila and human A-type lamins
Abstract
Nuclear intermediate filament proteins, called lamins, form a meshwork that lines the inner surface of the nuclear envelope. Lamins contain three domains: an N-terminal head, a central rod and a C-terminal tail domain possessing an Ig-fold structural motif. Lamins are classified as either A- or B-type based on structure and expression pattern. The Drosophila genome possesses two genes encoding lamins, Lamin C and lamin Dm(0), which have been designated A- and B-type, respectively, based on their expression profile and structural features. In humans, mutations in the gene encoding A-type lamins are associated with a spectrum of predominantly tissue-specific diseases known as laminopathies. Linking the disease phenotypes to cellular functions of lamins has been a major challenge. Drosophila is being used as a model system to identify the roles of lamins in development. Towards this end, we performed a comparative study of Drosophila and human A-type lamins. Analysis of transgenic flies showed that human lamins localize predictably within the Drosophila nucleus. Consistent with this finding, yeast two-hybrid data demonstrated conservation of partner-protein interactions. Drosophila lacking A-type lamin show nuclear envelope defects similar to those observed with human laminopathies. Expression of mutant forms of the A-type Drosophila lamin modeled after human disease-causing amino acid substitutions revealed an essential role for the N-terminal head and the Ig-fold in larval muscle tissue. This tissue-restricted sensitivity suggests a conserved role for lamins in muscle biology. In conclusion, we show that (1) localization of A-type lamins and protein-partner interactions are conserved between Drosophila and humans, (2) loss of the Drosophila A-type lamin causes nuclear defects and (3) muscle tissue is sensitive to the expression of mutant forms of A-type lamin modeled after those causing disease in humans. These studies provide new insights on the role of lamins in nuclear biology and support Drosophila as a model for studies of human laminopathies involving muscle dysfunction.
Conflict of interest statement
Figures
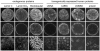



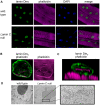

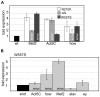
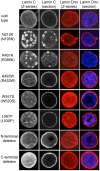
References
-
- Mattout A, Dechat T, Adam SA, Goldman RD, Gruenbaum Y. Nuclear lamins, diseases and aging. Curr Opin Cell Biol. 2006;18:335–341. - PubMed
-
- Misteli T, Scaffidi P. Genome instability in progeria: when repair gets old. Nat Med. 2005;11:718–719. - PubMed
-
- Foster HA, Stokes P, Forsey K, Leese HJ, Bridger JM. Lamins A and C are present in the nuclei of early porcine embryos, with lamin A being distributed in large intranuclear foci. Chromosome Res. 2007;15:163–174. - PubMed
-
- Rober RA, Weber K, Osborn M. Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development. 1989;105:365–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

