No effect of autologous growth factors (AGF) around ungrafted loaded implants in dogs
- PMID: 19856178
- PMCID: PMC2989027
- DOI: 10.1007/s00264-009-0897-8
No effect of autologous growth factors (AGF) around ungrafted loaded implants in dogs
Abstract
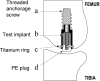
Autologous growth factors (AGF) is a growth-factor-rich concentrate of platelets, white blood cells and fibrinogen. Application of AGF was presumed to improve implant fixation and gap healing of non-grafted, loaded implants. We inserted one loaded titanium implant intra-articularly in each medial femoral condyle of eight dogs. Each implant was surrounded by a 0.75 mm gap. One implant in each dog was coated with AGF prior to implantation whereas the contralateral implant served as a control. AGF was prepared by isolating the buffy-coat from blood and further concentrated using an Interpore Cross UltraConcentrator. Platelet counts were increased from a median baseline of 168x10(3)/microl to 1003x10(3)/microl in AGF. However, AGF had no significant effect on implant fixation or bone formation. Even though AGF increased ultimate shear strength and energy absorption by approximately 50%, these differences had a p-value less than 0.05. The sample size in this study was small and any negative conclusions should be taken with caution due to low statistical power. We have previously demonstrated that AGF significantly improves fixation and incorporation of grafted implants. AGF might require mixing with an osteoconductive grafting material in order to provide a scaffold on which to foster bone growth and to keep the growth factors on location for a prolonged period.
Figures

References
-
- Elmengaard B, Bechtold JE, Soballe K (2005) In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J Biomed Mater Res A 75(2):249–255 - PubMed
-
- Gotfredsen K, Budtz-Jørgensen E, Jensen LL. Preparation and staining of sections containing titanium-implants. Stain Technol. 1989;64:121–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

