Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells
- PMID: 19856314
- PMCID: PMC2874465
- DOI: 10.1002/ijc.24988
Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells
Abstract
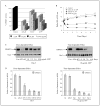
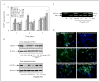
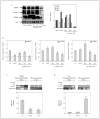
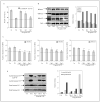
Epigenetic silencing of gluthathione-S-transferase pi (GSTP1) is recognized as being a molecular hallmark of human prostate cancer. We investigated the effects of green tea polyphenols (GTPs) on GSTP1 re-expression and further elucidated its mechanism of action and long-term safety, compared with nucleoside-analog inhibitor of DNA methyltransferase (DNMT), 5-aza-2'-deoxycitidine. Exposure of human prostate cancer LNCaP cells to 1-10 microg/ml of GTP for 1-7 days caused a concentration- and time-dependent re-expression of GSTP1, which correlated with DNMT1 inhibition. Methyl-specific-PCR and sequencing revealed extensive demethylation in the proximal GSTP1 promoter and regions distal to the transcription factor binding sites. GTP exposure in a time-dependent fashion diminished the mRNA and protein levels of MBD1, MBD4 and MeCP2; HDAC 1-3 and increased the levels of acetylated histone H3 (LysH9/18) and H4. Chromatin immunoprecipitation assays demonstrated that cells treated with GTP have reduced MBD2 association with accessible Sp1 binding sites leading to increased binding and transcriptional activation of the GSTP1 gene. Exposure of cells to GTP did not result in global hypomethylation, as demonstrated by methyl-specific PCR for LINE-1 promoter; rather GTP promotes maintenance of genomic integrity. Furthermore, exposure of cells to GTP did not cause activation of the prometaststic gene S100P, a reverse response noted after exposure of cells to 5-aza-2'deoxycitidine. Our results, for the first time, demonstrate that GTP has dual potential to alter DNA methylation and chromatin modeling, the 2 global epigenetic mechanisms of gene regulation and their lack of toxicity makes them excellent candidates for the chemoprevention of prostate cancer.
Figures

Similar articles
-
Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells.Carcinogenesis. 2008 Nov;29(11):2175-81. doi: 10.1093/carcin/bgn179. Epub 2008 Aug 1. Carcinogenesis. 2008. PMID: 18676679 Free PMC article.
-
Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer.Mol Carcinog. 2007 Jan;46(1):24-31. doi: 10.1002/mc.20258. Mol Carcinog. 2007. PMID: 16921492
-
Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells.Cancer Res. 2001 Jun 15;61(12):4820-6. Cancer Res. 2001. PMID: 11406558
-
GSTP1 DNA methylation and expression status is indicative of 5-aza-2'-deoxycytidine efficacy in human prostate cancer cells.PLoS One. 2011;6(9):e25634. doi: 10.1371/journal.pone.0025634. Epub 2011 Sep 28. PLoS One. 2011. PMID: 21980513 Free PMC article.
-
Lack of evidence for green tea polyphenols as DNA methylation inhibitors in murine prostate.Cancer Prev Res (Phila). 2009 Dec;2(12):1065-75. doi: 10.1158/1940-6207.CAPR-09-0010. Epub 2009 Nov 24. Cancer Prev Res (Phila). 2009. PMID: 19934341 Free PMC article.
Cited by
-
Methyl-Donors Can Induce Apoptosis and Attenuate Both the Akt and the Erk1/2 Mediated Proliferation Pathways in Breast and Lung Cancer Cell Lines.Int J Mol Sci. 2021 Mar 30;22(7):3598. doi: 10.3390/ijms22073598. Int J Mol Sci. 2021. PMID: 33808426 Free PMC article.
-
The Effects of Green Tea Catechins in Hematological Malignancies.Pharmaceuticals (Basel). 2023 Jul 18;16(7):1021. doi: 10.3390/ph16071021. Pharmaceuticals (Basel). 2023. PMID: 37513933 Free PMC article. Review.
-
HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase pi in prostate carcinoma cells.Mol Cancer Res. 2011 Jun;9(6):733-45. doi: 10.1158/1541-7786.MCR-10-0505. Epub 2011 May 26. Mol Cancer Res. 2011. PMID: 21622623 Free PMC article.
-
Perspectives on the recent developments with green tea polyphenols in drug discovery.Expert Opin Drug Discov. 2018 Jul;13(7):643-660. doi: 10.1080/17460441.2018.1465923. Epub 2018 Apr 24. Expert Opin Drug Discov. 2018. PMID: 29688074 Free PMC article. Review.
-
Sulforaphane and iberin are potent epigenetic modulators of histone acetylation and methylation in malignant melanoma.Eur J Nutr. 2021 Feb;60(1):147-158. doi: 10.1007/s00394-020-02227-y. Epub 2020 Mar 25. Eur J Nutr. 2021. PMID: 32215717
References
-
- Singal R, van Wert J, Bashambu M. Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. Cancer Res. 2001;61:4820–6. - PubMed
-
- Henderson CJ, McLaren AW, Moffat GJ, Bacon EJ, Wolf CR. Pi-class glutathione S-transferase: regulation and function. Chem Biol Interact. 1998;111–112:69–82. - PubMed
-
- Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–6. - PubMed
-
- Gsur A, Haidinger G, Hinteregger S, Bernhofer G, Schatzl G, Madersbacher S, Marberger M, Vutuc C, Micksche M. Polymorphisms of glutathione-S-transferase genes (GSTP1, GSTM1 and GSTT1) and prostate-cancer risk. Int J Cancer. 2001;95:152–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

