Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes
- PMID: 19856978
- PMCID: PMC2770093
- DOI: 10.1021/nn900947a
Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes
Erratum in
- ACS Nano. 2010 Mar 23;4(3):1769-70
Abstract
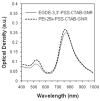
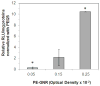
The propensity of nanoparticles to aggregate in aqueous media hinders their effective use in biomedical applications. Gold nanorods (GNRs) have been investigated as therapeutics, imaging agents, and diagnostics. We report that chemically generated gold nanorods rapidly aggregate in biologically relevant media. Depositing polyelectrolyte multilayers on gold nanorods enhanced the stability of these nanoparticles for at least up to 4 weeks. Dispersions of polyelectrolyte (PE)-gold nanorod assemblies (PE-GNRs) demonstrate a stable Arrhenius-like photothermal response, which was exploited for the hyperthermic ablation of prostate cancer cells in vitro. Subtoxic concentrations of PE-GNR assemblies were also employed for delivering exogenous plasmid DNA to prostate cancer cells. PE-GNRs based on a cationic polyelectrolyte recently synthesized in our laboratory demonstrated higher transfection efficacy and lower cytotoxicity compared to those based on polyethyleneimine, a current standard for polymer-mediated gene delivery. Our results indicate that judicious engineering of biocompatible polyelectrolytes leads to multifunctional gold nanorod-based assemblies that combine high stability and low cytotoxicity with photothermal ablation, gene delivery, and optical imaging capabilities on a single platform.
Figures












References
-
- Jabr-Milane L, van Vlerken L, Devalapally H, Shenoy D, Komareddy S, Bhavsar M, Amiji M. Multi-functional nanocarriers for targeted delivery of drugs and genes. J Control Release. 2008;130:121–128. - PubMed
-
- Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

