The aporphine alkaloid boldine induces adiponectin expression and regulation in 3T3-L1 cells
- PMID: 19857072
- PMCID: PMC3145162
- DOI: 10.1089/jmf.2008.0230
The aporphine alkaloid boldine induces adiponectin expression and regulation in 3T3-L1 cells
Abstract
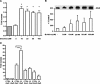
Adiponectin is an adipokine secreted by differentiated adipocytes. Clinical studies suggest a negative correlation between oxidative stress and adiponectin levels in patients with metabolic syndrome or cardiovascular disease. Natural compounds that can prevent oxidative stress mediated inhibition of adiponectin may be potentially therapeutic. Boldine, an aporphine alkaloid abundant in the medicinal plant Peumus boldus, is a powerful antioxidant. The current study demonstrates the effects of boldine on the expression of adiponectin and its regulators, CCAAT/enhancer binding protein-alpha (C/EBPalpha) and peroxisome proliferator-activated receptor (PPAR)-gamma, in 3T3-L1 cells. Differentiated 3T3-L1 adipocytes were exposed to either hydrogen peroxide (H(2)O(2)) (100 microM) or tumor necrosis factor-alpha (TNFalpha) (1 ng/mL) for 24 hours in the presence or absence of increasing concentrations of boldine (5-100 microM). Quantitative polymerase chain reaction showed that both the oxidants decreased the mRNA levels of adiponectin, PPARgamma, and C/EBPalpha to half of the control levels. Boldine, at all concentrations, counteracted the inhibitory effect of H(2)O(2) or TNFalpha and increased the expression of adiponectin and its regulators. The effect of boldine on adiponectin expression was biphasic, with the lower concentrations (5-25 microM) having a larger inductive effect compared to higher concentrations (50-100 microM). Boldine treatment alone in the absence of H(2)O(2) or TNFalpha was also able to induce adiponectin at the inductive phase of adipogenesis. Peroxisome proliferator response element-luciferase promoter transactivity analysis showed that boldine interacts with the PPAR response element and could potentially modulate PPAR responsive genes. Our results indicate that boldine is able to modulate the expression of adiponectin and its regulators in 3T3-L1 cells and has the potential to be beneficial in obesity-related cardiovascular disease.
Figures
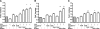



Similar articles
-
Mulberry leaf extract increases adiponectin in murine 3T3-L1 adipocytes.Nutr Res. 2012 Jan;32(1):39-44. doi: 10.1016/j.nutres.2011.12.003. Nutr Res. 2012. PMID: 22260862
-
Curcumin inhibits adipogenesis induced by benzyl butyl phthalate in 3T3-L1 cells.Toxicol Appl Pharmacol. 2017 Aug 15;329:158-164. doi: 10.1016/j.taap.2017.05.036. Toxicol Appl Pharmacol. 2017. PMID: 28595985
-
Kahweol inhibits adipogenesis of 3T3-L1 adipocytes through downregulation of PPARγ.Nat Prod Res. 2018 May;32(10):1216-1219. doi: 10.1080/14786419.2017.1326039. Epub 2017 May 16. Nat Prod Res. 2018. PMID: 28508719
-
An Overview of Chemistry, Kinetics, Toxicity and Therapeutic Potential of Boldine in Neurological Disorders.Neurochem Res. 2023 Nov;48(11):3283-3295. doi: 10.1007/s11064-023-03992-y. Epub 2023 Jul 18. Neurochem Res. 2023. PMID: 37462836 Review.
-
Boldine Ameliorates Vascular Oxidative Stress and Endothelial Dysfunction: Therapeutic Implication for Hypertension and Diabetes.J Cardiovasc Pharmacol. 2015 Jun;65(6):522-31. doi: 10.1097/FJC.0000000000000185. J Cardiovasc Pharmacol. 2015. PMID: 25469805 Free PMC article. Review.
Cited by
-
Anti-hyperglycemic and anti-hyperlipidemia effects of the alkaloid-rich extract from barks of Litsea glutinosa in ob/ob mice.Sci Rep. 2018 Aug 23;8(1):12646. doi: 10.1038/s41598-018-30823-w. Sci Rep. 2018. PMID: 30140027 Free PMC article.
-
Inhibition of Gluconeogenesis by Boldine in the Perfused Liver: Therapeutical Implication for Glycemic Control.Int J Hepatol. 2023 Apr 4;2023:1283716. doi: 10.1155/2023/1283716. eCollection 2023. Int J Hepatol. 2023. PMID: 37056327 Free PMC article.
-
Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds.Mol Biol Rep. 2021 Jan;48(1):743-761. doi: 10.1007/s11033-020-06036-8. Epub 2020 Dec 4. Mol Biol Rep. 2021. PMID: 33275195 Review.
-
Natural Aporphine Alkaloids with Potential to Impact Metabolic Syndrome.Molecules. 2021 Oct 10;26(20):6117. doi: 10.3390/molecules26206117. Molecules. 2021. PMID: 34684698 Free PMC article. Review.
-
Evaluation of structural effects on 5-HT(2A) receptor antagonism by aporphines: identification of a new aporphine with 5-HT(2A) antagonist activity.Bioorg Med Chem Lett. 2014 Apr 1;24(7):1664-7. doi: 10.1016/j.bmcl.2014.02.066. Epub 2014 Mar 4. Bioorg Med Chem Lett. 2014. PMID: 24630561 Free PMC article.
References
-
- Hu E. Liang P. Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. - PubMed
-
- Scherer PE. Williams S. Fogliano M. Baldini G. Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. - PubMed
-
- Lara-Castro C. Fu Y. Chung BH. Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. - PubMed
-
- Sheng T. Yang K. Adiponectin and its association with insulin resistance and type 2 diabetes. J Genet Genomics. 2008;35:321–326. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

