Present and future arboviral threats
- PMID: 19857523
- PMCID: PMC2815176
- DOI: 10.1016/j.antiviral.2009.10.008
Present and future arboviral threats
Abstract
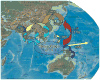
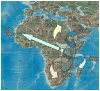
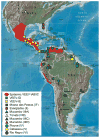
Arthropod-borne viruses (arboviruses) are important causes of human disease nearly worldwide. All arboviruses circulate among wild animals, and many cause disease after spillover transmission to humans and agriculturally important domestic animals that are incidental or dead-end hosts. Viruses such as dengue (DENV) and chikungunya (CHIKV) that have lost the requirement for enzootic amplification now produce extensive epidemics in tropical urban centers. Many arboviruses recently have increased in importance as human and veterinary pathogens using a variety of mechanisms. Beginning in 1999, West Nile virus (WNV) underwent a dramatic geographic expansion into the Americas. High amplification associated with avian virulence coupled with adaptation for replication at higher temperatures in mosquito vectors, has caused the largest epidemic of arboviral encephalitis ever reported in the Americas. Japanese encephalitis virus (JEV), the most frequent arboviral cause of encephalitis worldwide, has spread throughout most of Asia and as far south as Australia from its putative origin in Indonesia and Malaysia. JEV has caused major epidemics as it invaded new areas, often enabled by rice culture and amplification in domesticated swine. Rift Valley fever virus (RVFV), another arbovirus that infects humans after amplification in domesticated animals, undergoes epizootic transmission during wet years following droughts. Warming of the Indian Ocean, linked to the El Niño-Southern Oscillation in the Pacific, leads to heavy rainfall in east Africa inundating surface pools and vertically infected mosquito eggs laid during previous seasons. Like WNV, JEV and RVFV could become epizootic and epidemic in the Americas if introduced unintentionally via commerce or intentionally for nefarious purposes. Climate warming also could facilitate the expansion of the distributions of many arboviruses, as documented for bluetongue viruses (BTV), major pathogens of ruminants. BTV, especially BTV-8, invaded Europe after climate warming and enabled the major midge vector to expand is distribution northward into southern Europe, extending the transmission season and vectorial capacity of local midge species. Perhaps the greatest health risk of arboviral emergence comes from extensive tropical urbanization and the colonization of this expanding habitat by the highly anthropophilic (attracted to humans) mosquito, Aedes aegypti. These factors led to the emergence of permanent endemic cycles of urban DENV and CHIKV, as well as seasonal interhuman transmission of yellow fever virus. The recent invasion into the Americas, Europe and Africa by Aedes albopictus, an important CHIKV and secondary DENV vector, could enhance urban transmission of these viruses in tropical as well as temperate regions. The minimal requirements for sustained endemic arbovirus transmission, adequate human viremia and vector competence of Ae. aegypti and/or Ae. albopictus, may be met by two other viruses with the potential to become major human pathogens: Venezuelan equine encephalitis virus, already an important cause of neurological disease in humans and equids throughout the Americas, and Mayaro virus, a close relative of CHIKV that produces a comparably debilitating arthralgic disease in South America. Further research is needed to understand the potential of these and other arboviruses to emerge in the future, invade new geographic areas, and become important public and veterinary health problems.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures

References
-
- Outbreak news. Yellow fever, Paraguay. Wkly Epidemiol Rec. 2008;83:105. - PubMed
-
- Adekolu-John EO, Fagbami AH. Arthropod-borne virus antibodies in sera of residents of Kainji Lake Basin, Nigeria 1980. Trans R Soc Trop Med Hyg. 1983;77:149–51. - PubMed
-
- Aguilar PV, Greene IP, Coffey LL, Medina G, Moncayo AC, Anishchenko M, Ludwig GV, Turell MJ, O’Guinn ML, Lee J, Tesh RB, Watts DM, Russell KL, Hice C, Yanoviak S, Morrison AC, Klein TA, Dohm DJ, Guzman H, Travassos da Rosa AP, Guevara C, Kochel T, Olson J, Cabezas C, Weaver SC. Endemic Venezuelan equine encephalitis in northern Peru. Emerg Infect Dis. 2004;10:880–8. - PMC - PubMed
-
- Anderson JF, Andreadis TG, Main AJ, Ferrandino FJ, Vossbrinck CR. West Nile virus from female and male mosquitoes (Diptera: Culicidae) in subterranean, ground, and canopy habitats in Connecticut. J Med Entomol. 2006;43:1010–9. - PubMed
-
- Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae) J Med Entomol. 2008;45:445–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

