Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids
- PMID: 19857569
- PMCID: PMC2818734
- DOI: 10.1016/j.freeradbiomed.2009.10.046
Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids
Abstract
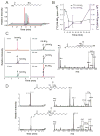
Reactive oxygen species mediate a decrease in nitric oxide (NO) bioavailability and endothelial dysfunction, with secondary oxidized and nitrated by-products of these reactions contributing to the pathogenesis of numerous vascular diseases. While oxidized lipids and lipoproteins exacerbate inflammatory reactions in the vasculature, in stark contrast the nitration of polyunsaturated fatty acids and complex lipids yields electrophilic products that exhibit pluripotent anti-inflammatory signaling capabilities acting via both cGMP-dependent and -independent mechanisms. Herein we report that nitro-oleic acid (OA-NO(2)) treatment increases expression of endothelial nitric oxide synthase (eNOS) and heme oxygenase 1 (HO-1) in the vasculature, thus transducing vascular protective effects associated with enhanced NO production. Administration of OA-NO(2) via osmotic pump results in a significant increase in eNOS and HO-1 mRNA in mouse aortas. Moreover, HPLC-MS/MS analysis showed that NO(2)-FAs are rapidly metabolized in cultured endothelial cells (ECs) and treatment with NO(2)-FAs stimulated the phosphorylation of eNOS at Ser(1179). These posttranslational modifications of eNOS, in concert with elevated eNOS gene expression, contributed to an increase in endothelial NO production. In aggregate, OA-NO(2)-induced eNOS and HO-1 expression by vascular cells can induce beneficial effects on endothelial function and provide a new strategy for treating various vascular inflammatory and hypertensive disorders.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures


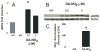


References
-
- Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280:19289–19297. - PubMed
-
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

