A bifunctional regulatory element in human somatic Wee1 mediates cyclin A/Cdk2 binding and Crm1-dependent nuclear export
- PMID: 19858290
- PMCID: PMC2798281
- DOI: 10.1128/MCB.01876-08
A bifunctional regulatory element in human somatic Wee1 mediates cyclin A/Cdk2 binding and Crm1-dependent nuclear export
Abstract
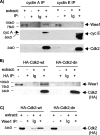
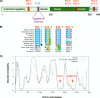
Sophisticated models for the regulation of mitotic entry are lacking for human cells. Inactivating human cyclin A/Cdk2 complexes through diverse approaches delays mitotic entry and promotes inhibitory phosphorylation of Cdk1 on tyrosine 15, a modification performed by Wee1. We show here that cyclin A/Cdk2 complexes physically associate with Wee1 in U2OS cells. Mutation of four conserved RXL cyclin A/Cdk binding motifs (RXL1 to RXL4) in Wee1 diminished stable binding. RXL1 resides within a large regulatory region of Wee1 that is predicted to be intrinsically disordered (residues 1 to 292). Near RXL1 is T239, a site of inhibitory Cdk phosphorylation in Xenopus Wee1 proteins. We found that T239 is phosphorylated in human Wee1 and that this phosphorylation was reduced in an RXL1 mutant. RXL1 and T239 mutants each mediated greater Cdk phosphorylation and G(2)/M inhibition than the wild type, suggesting that cyclin A/Cdk complexes inhibit human Wee1 through these sites. The RXL1 mutant uniquely also displayed increased nuclear localization. RXL1 is embedded within sequences homologous to Crm1-dependent nuclear export signals (NESs). Coimmunoprecipitation showed that Crm1 associated with Wee1. Moreover, treatment with the Crm1 inhibitor leptomycin B or independent mutation of the potential NES (NESm) abolished Wee1 nuclear export. Export was also reduced by Cdk inhibition or cyclin A RNA interference, suggesting that cyclin A/Cdk complexes contribute to Wee1 export. Somewhat surprisingly, NESm did not display increased G(2)/M inhibition. Thus, nuclear export of Wee1 is not essential for mitotic entry though an important functional role remains likely. These studies identify a novel bifunctional regulatory element in Wee1 that mediates cyclin A/Cdk2 association and nuclear export.
Figures










References
-
- Baldin, V., and B. Ducommun. 1995. Subcellular localisation of human wee1 kinase is regulated during the cell cycle. J. Cell Sci. 108:2425-2432. - PubMed
-
- Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 13:1775-1785. - PubMed
-
- Booher, R. N., P. S. Holman, and A. Fattaey. 1997. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J. Biol. Chem. 272:22300-22306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
