Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen
- PMID: 19858303
- PMCID: PMC2798189
- DOI: 10.1128/IAI.00691-09
Functional characterization of Borrelia spielmanii outer surface proteins that interact with distinct members of the human factor H protein family and with plasminogen
Abstract
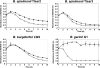
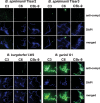
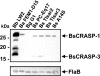
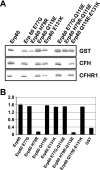
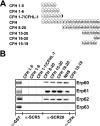
Acquisition of complement regulator factor H (CFH) and factor H-like protein 1 (CFHL1) from human serum enables Borrelia spielmanii, one of the etiological agents of Lyme disease, to evade complement-mediated killing by the human host. Up to three distinct complement regulator-acquiring surface proteins (CRASPs) may be expressed by serum-resistant B. spielmanii, each exhibiting an affinity for CFH and/or CFHL1. Here, we describe the functional characterization of the 15-kDa CRASPs of B. spielmanii, members of the polymorphic Erp (OspE/F-related) protein family, that bind two distinct host complement regulators, CFH and factor H-related protein 1 (CFHR1), but not CFHL1. CFH bound to the B. spielmanii CRASPs maintained cofactor activity for factor I-mediated C3b inactivation. Three naturally occurring alleles of this protein bound CFH and CFHR1 while a fourth natural allele could not. Comparative sequence analysis of these protein alleles identified a single amino acid, histidine-79, as playing a significant role in CFH/CFHR1 binding, with substitution by an arginine completely abrogating ligand binding. The mutation of His-79 to Arg did not inhibit binding of plasminogen, another known ligand of this group of borrelial outer-surface proteins.
Figures



References
-
- Alitalo, A., T. Meri, T. Chen, H. Lankinen, Z.-Z. Cheng, T. S. Jokiranta, I. J. T. Seppala, P. Lahdenne, P. S. Hefty, D. R. Akins, and S. Meri. 2004. Lysine-dependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface protein E to the C terminus of factor H. J. Immunol. 172:6195-6201. - PubMed
-
- Alitalo, A., T. Meri, P. Comstedt, L. Jeffery, J. Tornberg, T. Strandin, H. Lankinen, S. Bergström, M. Cinco, S. R. Vuppala, D. R. Akins, and S. Meri. 2005. Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur. J. Immunol. 35:3043-3053. - PubMed
-
- Alitalo, A., T. Meri, H. Lankinen, I. Seppala, P. Lahdenne, P. S. Hefty, D. Akins, and S. Meri. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847-3853. - PubMed
-
- Areschoug, T., M. Stalhammar-Carlemalm, I. Karlsson, and G. Lindahl. 2002. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 277:12642-12648. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

