Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies
- PMID: 19858307
- PMCID: PMC2798211
- DOI: 10.1128/IAI.00497-09
Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies
Abstract
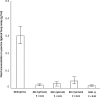
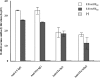
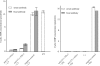
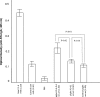
Enterotoxigenic Escherichia coli (ETEC) strains are a major cause of diarrheal disease in humans and farm animals. E. coli fimbriae, or colonization factor antigens (CFAs), and enterotoxins, including heat-labile enterotoxins (LT) and heat-stable enterotoxins (ST), are the key virulence factors in ETEC diarrhea. Unlike fimbriae or LT, STa has not often been included as an antigen in development of vaccines against ETEC diarrhea because of its poor immunogenicity. STa becomes immunogenic only after being coupled with a strongly immunogenic carrier protein. However, native or shorter STa antigens either had to retain toxic activity in order to become antigenic or elicited anti-STa antibodies that were not sufficiently protective. In this study, we genetically mutated the porcine LT (pLT) gene for a pLT(192(R-->G)) toxoid and the porcine STa (pSTa) gene for three full-length pSTa toxoids [STa(11(N-->K)), STa(12(P-->F)), and STa(13(A-->Q))] and used the full-length pLT(192) as an adjuvant to carry the pSTa toxoid for pLT(192):pSTa-toxoid fusion antigens. Rabbits immunized with pLT(192):pSTa(12) or pLT(192):pSTa(13) fusion protein developed high titers of anti-LT and anti-STa antibodies. Furthermore, rabbit antiserum and antifecal antibodies were able to neutralize purified cholera toxin (CT) and STa toxin. In addition, preliminary data suggested that suckling piglets born by a sow immunized with the pLT(192):pSTa(13) fusion antigen were protected when challenged with an STa-positive ETEC strain. This study demonstrated that pSTa toxoids are antigenic when fused with a pLT toxoid and that the elicited anti-LT and anti-STa antibodies were protective. This fusion strategy could provide instructive information to develop effective toxoid vaccines against ETEC-associated diarrhea in animals and humans.
Figures



References
-
- Alexander, T. J. L. 1994. Neonatal diarrhoea in pigs, p. 151-170. In C. L. Gyles (ed.), Escherichia coli in domestic animals and humans. CAB International, Oxon, United Kingdom.
-
- Arakawa, R., J. Yu, and W. H. R. Langridge. 2001. Synthesis of a cholera toxin B subunit-rotavirus NSP4 fusion protein in potato. Plant Cell Rep. 20:343-348. - PubMed
-
- Ausubel, F. M., R. Brent, R. K. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1999. Short protocols in molecular biology, 4th ed. John Wiley & Sons, Inc., New York, NY.
-
- Batisson, I., and M. Der Vartinian. 2000. Contribution of defined amino acid residues to the immunogenicity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins. FEMS Microbiol. Lett. 192:223-229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

