Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904
- PMID: 19858396
- PMCID: PMC2792952
- DOI: 10.1200/JCO.2009.22.1309
Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904
Abstract
Purpose: The relative value of gemcitabine-based combination chemotherapy therapy and prolonged infusions of gemcitabine in patients with advanced pancreatic cancer remains controversial. We explored the efficacy and toxicity of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in a multi-institutional, randomized, phase II study.
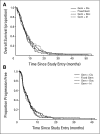
Patients and methods: Patients with metastatic pancreatic cancer were randomly assigned to one of the following four regimens: gemcitabine 1,000 mg/m(2) on days 1, 8, and 15 with cisplatin 50 mg/m(2) on days 1 and 15 (arm A); gemcitabine 1,500 mg/m(2) at a rate of 10 mg/m(2)/min on days 1, 8, and 15 (arm B); gemcitabine 1,000 mg/m(2) with docetaxel 40 mg/m(2) on days 1 and 8 (arm C); or gemcitabine 1,000 mg/m(2) with irinotecan 100 mg/m(2) on days 1 and 8 (arm D). Patients were observed for response, toxicity, and survival. Results Two hundred fifty-nine patients were enrolled onto the study, of whom 245 were eligible and received treatment. Anticipated rates of myelosuppression, fatigue, and expected regimen-specific toxicities were observed. The overall tumor response rates were 12% to 14%, and the median overall survival times were 6.4 to 7.1 months among the four regimens.
Conclusion: Gemcitabine/cisplatin, fixed dose rate gemcitabine, gemcitabine/docetaxel, and gemcitabine/irinotecan have similar antitumor activity in metastatic pancreatic cancer. In light of recent negative randomized studies directly comparing several of these regimens with standard gemcitabine, none of these approaches can be recommended for routine use in patients with this disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Changing the paradigm in conducting randomized clinical studies in advanced pancreatic cancer: an opportunity for better clinical development.J Clin Oncol. 2009 Nov 20;27(33):5487-91. doi: 10.1200/JCO.2009.23.3098. Epub 2009 Oct 26. J Clin Oncol. 2009. PMID: 19858387 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Burris H, Moore M, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. - PubMed
-
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. - PubMed
-
- Cunningham D, Chau I, Stocken D, et al. Phase III randomised comparison of gemcitabine with gemcitabine plus capecitabine in patients with advanced pancreatic cancer. Eur J Cancer. 2005;3(suppl):4.
-
- Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: A randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Res and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–2217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical