Antibiotic use in neonatal intensive care units and adherence with Centers for Disease Control and Prevention 12 Step Campaign to Prevent Antimicrobial Resistance
- PMID: 19858773
- PMCID: PMC4526135
- DOI: 10.1097/INF.0b013e3181b12484
Antibiotic use in neonatal intensive care units and adherence with Centers for Disease Control and Prevention 12 Step Campaign to Prevent Antimicrobial Resistance
Abstract
Background: The Centers for Disease Control and Prevention (CDC) 12-Step Campaign to Prevent Antimicrobial Resistance was launched to educate clinicians about antimicrobial resistance and provide strategies to improve clinical practice, including antimicrobial utilization.
Methods: A multicenter retrospective observational study of antibiotic use was performed in 4 tertiary care NICUs to assess adherence to the guidelines defined by the CDC 12-Step Campaign using predetermined criteria. Fifty infants per NICU were identified who received intravenous antibiotics at greater than 72 hours of age. Antibiotic regimens, clinical and microbiologic data, and indications for initiation and continuation of antibiotics (after 72 hours of use) were recorded. Inappropriate utilization was characterized at initiation, continuation, by agent, and by CDC 12-Step.
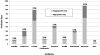
Results: Two hundred neonates received 323 antibiotic courses totaling 3344 antibiotic-days. Ninety (28%) courses and 806 (24%) days were judged to be nonadherent to a CDC 12-Step. Inappropriate use was more common with continuation of antibiotics (39%) than with initiation (4%) of therapy. Vancomycin was the most commonly used drug (n = 895 antibiotic-days) of which 284 (32%) days were considered inappropriate. Carbapenems were used less frequently (n = 310 antibiotic-days), and 132 (43%) of these days were inappropriate. Common reasons for nonadherence at the time of continuation included failure to narrow antibiotic coverage after microbiologic results were known and prolonged antibiotic prophylaxis after surgery with chest tube placement.
Conclusions: The CDC 12-Step Campaign can be modified for neonatal populations. Inappropriate antibiotic prescribing was common in the study NICUs. Improvement efforts should target antibiotic use 72 hours after initiation, particularly focusing on narrowing therapy and instituting protocols to limit prophylaxis.
Figures
References
-
- Centers for Disease Control and Prevention. 12-Step Program to Prevent Antimicrobial Resistance in Health Care Settings. 2002 Available at: http://www.cdc.gov/drugresistance/healthcare/default.html. Accessed 2008.
-
- Banerjee S, Grohskopf LA, Sinkowitz-Cochran RL, et al. National Nosocomial Infections Surveillance System. Pediatric Prevention Network Incidence of pediatric and neonatal intensive care unit-acquired infections. Infect Control Hosp Epidemiol. 2006;27:561–570. - PubMed
-
- Grohskopf L, Huskins WC, Sinkowitz-Cochran RL, et al. Use of Antimicrobial agents in US neonatal and pediatric intensive patients. Pediatr Infect Dis J. 2005;24:766–773. - PubMed
-
- Jones DA, Pulver BL, Tai B, et al. Glycopeptide prescribing in an Australian tertiary paediatric hospital. J Paediatr Child Health. 2001;37:342–347. - PubMed
-
- Dellinger PE, Gross PA, Barrett TL, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Clin Infect Dis. 1994;18:422–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical