Global conformational dynamics of a Y-family DNA polymerase during catalysis
- PMID: 19859523
- PMCID: PMC2758995
- DOI: 10.1371/journal.pbio.1000225
Global conformational dynamics of a Y-family DNA polymerase during catalysis
Abstract
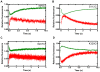
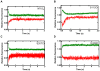
Replicative DNA polymerases are stalled by damaged DNA while the newly discovered Y-family DNA polymerases are recruited to rescue these stalled replication forks, thereby enhancing cell survival. The Y-family DNA polymerases, characterized by low fidelity and processivity, are able to bypass different classes of DNA lesions. A variety of kinetic and structural studies have established a minimal reaction pathway common to all DNA polymerases, although the conformational intermediates are not well defined. Furthermore, the identification of the rate-limiting step of nucleotide incorporation catalyzed by any DNA polymerase has been a matter of long debate. By monitoring time-dependent fluorescence resonance energy transfer (FRET) signal changes at multiple sites in each domain and DNA during catalysis, we present here a real-time picture of the global conformational transitions of a model Y-family enzyme: DNA polymerase IV (Dpo4) from Sulfolobus solfataricus. Our results provide evidence for a hypothetical DNA translocation event followed by a rapid protein conformational change prior to catalysis and a subsequent slow, post-chemistry protein conformational change. Surprisingly, the DNA translocation step was induced by the binding of a correct nucleotide. Moreover, we have determined the directions, rates, and activation energy barriers of the protein conformational transitions, which indicated that the four domains of Dpo4 moved in a synchronized manner. These results showed conclusively that a pre-chemistry conformational change associated with domain movements was too fast to be the rate-limiting step. Rather, the rearrangement of active site residues limited the rate of correct nucleotide incorporation. Collectively, the conformational dynamics of Dpo4 offer insights into how the inter-domain movements are related to enzymatic function and their concerted interactions with other proteins at the replication fork.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
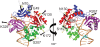




Similar articles
-
Mechanism of DNA polymerization catalyzed by Sulfolobus solfataricus P2 DNA polymerase IV.Biochemistry. 2004 Feb 24;43(7):2116-25. doi: 10.1021/bi035746z. Biochemistry. 2004. PMID: 14967051
-
Conformational dynamics of a Y-family DNA polymerase during substrate binding and catalysis as revealed by interdomain Förster resonance energy transfer.Biochemistry. 2014 Mar 25;53(11):1768-78. doi: 10.1021/bi5000146. Epub 2014 Mar 12. Biochemistry. 2014. PMID: 24568554 Free PMC article.
-
Effect of N2-guanyl modifications on early steps in catalysis of polymerization by Sulfolobus solfataricus P2 DNA polymerase Dpo4 T239W.J Mol Biol. 2010 Feb 5;395(5):1007-18. doi: 10.1016/j.jmb.2009.11.071. Epub 2009 Dec 4. J Mol Biol. 2010. PMID: 19969000 Free PMC article.
-
Recent insight into the kinetic mechanisms and conformational dynamics of Y-Family DNA polymerases.Biochemistry. 2014 May 6;53(17):2804-14. doi: 10.1021/bi5000405. Epub 2014 Apr 23. Biochemistry. 2014. PMID: 24716482 Free PMC article. Review.
-
Regulation of DNA repair fidelity by molecular checkpoints: "gates" in DNA polymerase beta's substrate selection.Biochemistry. 2006 Dec 26;45(51):15142-56. doi: 10.1021/bi061353z. Epub 2006 Dec 1. Biochemistry. 2006. PMID: 17176036 Free PMC article. Review.
Cited by
-
Noncognate DNA damage prevents the formation of the active conformation of the Y-family DNA polymerases DinB and DNA polymerase κ.FEBS J. 2015 Jul;282(14):2646-60. doi: 10.1111/febs.13304. Epub 2015 May 11. FEBS J. 2015. PMID: 25899385 Free PMC article.
-
Unlocking the sugar "steric gate" of DNA polymerases.Biochemistry. 2011 Feb 22;50(7):1135-42. doi: 10.1021/bi101915z. Epub 2011 Jan 26. Biochemistry. 2011. PMID: 21226515 Free PMC article. Review.
-
Protein Ensembles: How Does Nature Harness Thermodynamic Fluctuations for Life? The Diverse Functional Roles of Conformational Ensembles in the Cell.Chem Rev. 2016 Jun 8;116(11):6516-51. doi: 10.1021/acs.chemrev.5b00562. Epub 2016 Jan 25. Chem Rev. 2016. PMID: 26807783 Free PMC article. Review.
-
Single-molecule investigation of substrate binding kinetics and protein conformational dynamics of a B-family replicative DNA polymerase.J Biol Chem. 2013 Apr 19;288(16):11590-600. doi: 10.1074/jbc.M113.459982. Epub 2013 Mar 5. J Biol Chem. 2013. PMID: 23463511 Free PMC article.
-
Investigating the Conformational Dynamics of a Y-Family DNA Polymerase during Its Folding and Binding to DNA and a Nucleotide.JACS Au. 2021 Dec 16;2(2):341-356. doi: 10.1021/jacsau.1c00368. eCollection 2022 Feb 28. JACS Au. 2021. PMID: 35252985 Free PMC article.
References
-
- Henzler-Wildman K, Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. - PubMed
-
- Pelletier H, Sawaya M. R, Wolfle W, Wilson S. H, Kraut J. Crystal structures of human DNA polymerase beta complexed with DNA: implications for catalytic mechanism, processivity, and fidelity. Biochemistry. 1996;35:12742–12761. - PubMed
-
- Franklin M. C, Wang J, Steitz T. A. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell. 2001;105:657–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

