Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial
- PMID: 19859531
- PMCID: PMC2760761
- DOI: 10.1371/journal.pmed.1000172
Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: a randomized clinical trial
Abstract
Background: Single-dose nevirapine (sdNVP)-which prevents mother-to-child transmission of HIV-selects non-nucleoside reverse-transcriptase inhibitor (NNRTI) resistance mutations in the majority of women and HIV-infected infants receiving it. This open-label, randomised trial examined the efficacy of short-course zidovudine (AZT) and lamivudine (3TC) with sdNVP in reducing NNRTI resistance in mothers, and as a secondary objective, in infants, in a setting where sdNVP was standard-of-care.
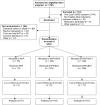
Methods and findings: sdNVP alone, administered at the onset of labour and to the infant, was compared to sdNVP with AZT plus 3TC, given as combivir (CBV) for 4 (NVP/CBV4) or 7 (NVP/CBV7) days, initiated simultaneously with sdNVP in labour; their newborns received the same regimens. Women were randomised 1ratio1ratio1. HIV-1 resistance was assessed by population sequencing at: baseline, 2, and 6 wk after birth. An unplanned interim analysis resulted in early stopping of the sdNVP arm. 406 pregnant women were randomised and took study medication (sdNVP 74, NVP/CBV4 164, and NVP/CBV7 168). HIV-1 resistance mutations emerged in 59.2%, 11.7%, and 7.3% of women in the sdNVP, NVP/CBV4, and NVP/CBV7 arms by 6 wk postpartum; differences between NVP-only and both NVP/CBV arms were significant (p<0.0001), but the difference between NVP/CBV4 and NVP/CBV7 was not (p = 0.27). Estimated efficacy comparing combined CBV arms with sdNVP was 85.6%. Similar resistance reductions were seen in infants who were HIV-infected by their 6-wk visit.
Conclusions: A short course of AZT plus 3TC, supplementing maternal and infant sdNVP, reduces emergent NNRTI resistance mutations in both mothers and their infants. However, this trial was not powered to detect small differences between the CBV arms.
Trial registration: www.ClinicalTrials.govNCT 00144183.
Trial registration: ClinicalTrials.gov NCT00144183.
Conflict of interest statement
M. Hopley, M. Ekelund, D. B. Hall, and P. Robinson are employed by Boehringer Ingelheim, the sponsor of the study, and D. Mayers was employed by Boehringer Ingelheim at the time of the study. Employees of the sponsor were involved in protocol design, were responsible for data management and statistical analyses, and assisted with the preparation of the paper. J. A. McIntyre and G. E. Gray have received research funding, travel grants and speaker's honoraria from Boehringer Ingelheim and Glaxo SmithKline. N. A. Martinson and D. Moodley declare no competing interests.
Figures
Comment in
-
Antiretroviral strategies to prevent mother-to-child transmission of HIV: striking a balance between efficacy, feasibility, and resistance.PLoS Med. 2009 Oct;6(10):e1000169. doi: 10.1371/journal.pmed.1000169. Epub 2009 Oct 27. PLoS Med. 2009. PMID: 19859532 Free PMC article.
References
-
- Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. - PubMed
-
- Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. - PubMed
-
- Cressey TR, Jourdain G, Lallemant MJ, Kunkeaw S, Jackson JB, et al. Persistence of nevirapine exposure during the postpartum period after intrapartum single-dose nevirapine in addition to zidovudine prophylaxis for the prevention of mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr. 2005;38:283–288. - PubMed
-
- Muro E, Droste JA, Hofstede HT, Bosch M, Dolmans W, et al. Nevirapine plasma concentrations are still detectable after more than 2 weeks in the majority of women receiving single-dose nevirapine: implications for intervention studies. J Acquir Immune Defic Syndr. 2005;39:419–421. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical