A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon
- PMID: 19859560
- PMCID: PMC2763199
- DOI: 10.1371/journal.pone.0007611
A randomized trial assessing the safety and immunogenicity of AS01 and AS02 adjuvanted RTS,S malaria vaccine candidates in children in Gabon
Abstract
Background: The malaria vaccine candidate antigen RTS,S includes parts of the pre-erythrocytic stage circumsporozoite protein fused to the Hepatitis B surface antigen. Two Adjuvant Systems are in development for this vaccine, an oil-in water emulsion--based formulation (AS02) and a formulation based on liposomes (AS01).
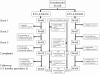
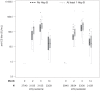
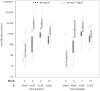
Methods & principal findings: In this Phase II, double-blind study (NCT00307021), 180 healthy Gabonese children aged 18 months to 4 years were randomized to receive either RTS,S/AS01(E) or RTS,S/AS02(D), on a 0-1-2 month vaccination schedule. The children were followed-up daily for six days after each vaccination and monthly for 14 months. Blood samples were collected at 4 time-points. Both vaccines were well tolerated. Safety parameters were distributed similarly between the two groups. Both vaccines elicited a strong specific immune response after Doses 2 and 3 with a ratio of anti-CS GMT titers (AS02(D)/AS01(E)) of 0.88 (95% CI: 0.68-1.15) post-Dose 3. After Doses 2 and 3 of experimental vaccines, anti-CS and anti-HBs antibody GMTs were higher in children who had been previously vaccinated with at least one dose of hepatitis B vaccine compared to those not previously vaccinated.
Conclusions: RTS,S/AS01(E) proved similarly as well tolerated and immunogenic as RTS,S/AS02(D), completing an essential step in the age de-escalation process within the RTS,S clinical development plan.
Trial registration: ClinicalTrials.gov. NCT00307021.
Conflict of interest statement
Figures
References
-
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg. 2004;71(2 suppl):1–15. - PubMed
-
- Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. - PubMed
-
- Girard MP, Reed ZH, Friede M, Kieny MP. A review of human vaccine research and development. Malaria Vaccine. 2007;25:1567–1580. - PubMed
-
- Malkin E, Dubovsky F, Moree M. Progress towards the development of malaria vaccines. Trends Parasitol. 2006;22:292–295. - PubMed
-
- Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: Single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

