An overview of RNAs with regulatory functions in gram-positive bacteria
- PMID: 19859665
- PMCID: PMC11115938
- DOI: 10.1007/s00018-009-0162-8
An overview of RNAs with regulatory functions in gram-positive bacteria
Abstract
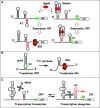
During the last decade, RNA molecules with regulatory functions on gene expression have benefited from a renewed interest. In bacteria, recent high throughput computational and experimental approaches have led to the discovery that 10-20% of all genes code for RNAs with critical regulatory roles in metabolic, physiological and pathogenic processes. The trans-acting RNAs comprise the noncoding RNAs, RNAs with a short open reading frame and antisense RNAs. Many of these RNAs act through binding to their target mRNAs while others modulate protein activity or target DNA. The cis-acting RNAs include regulatory regions of mRNAs that can respond to various signals. These RNAs often provide the missing link between sensing changing conditions in the environment and fine-tuning the subsequent biological responses. Information on their various functions and modes of action has been well documented for gram-negative bacteria. Here, we summarize the current knowledge of regulatory RNAs in gram-positive bacteria.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

