Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data
- PMID: 19859937
- PMCID: PMC4275127
- DOI: 10.1002/mrm.22135
Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data
Abstract
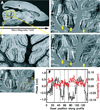
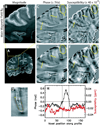
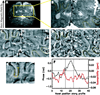
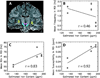
Phase images in susceptibility-weighted MRI of brain provide excellent contrast. However, the phase is affected by tissue geometry and orientation relative to the main magnetic field (B(0)), and phase changes extend beyond areas of altered susceptibility. Magnetic susceptibility, on the other hand, is an intrinsic tissue property, closely reflecting tissue composition. Therefore, recently developed inverse Fourier-based methods were applied to calculate susceptibility maps from high-resolution phase images acquired at a single orientation at 7 T in the human brain (in vivo and fixed) and at 11.7 T in fixed marmoset brain. In susceptibility images, the contrast of cortical layers was more consistent than in phase images and was independent of the structures' orientation relative to B(0). The contrast of iron-rich deep-brain structures (red nucleus and substantia nigra) in susceptibility images agreed more closely with iron-dominated R(2) (*) images than the phase image contrast, which extended outside the structures. The mean susceptibility in these regions was significantly correlated with their estimated iron content. Susceptibility maps calculated using this method overcome the orientation-dependence and non-locality of phase image contrast and seem to reflect underlying tissue composition. Susceptibility images should be easier to interpret than phase images and could improve our understanding of the sources of susceptibility contrast.
(c) 2009 Wiley-Liss, Inc.
Figures



References
-
- Bourekas EC, Christoforidis GA, Abduljalil AM, Kangarlu A, Chakeres DW, Spigos DG, Robitaille PM. High resolution MRI of the deep gray nuclei at 8 Tesla. J Comput Assist Tomogr. 1999;23(6):867–874. - PubMed
-
- Ogg RJ, Langston JW, Haacke EM, Steen RG, Taylor JS. The correlation between phase shifts in gradient-echo MR images and regional brain iron concentration. Magn Reson Imaging. 1999;17(8):1141–1148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

