UGT1A1 gene polymorphism: impact on toxicity and efficacy of irinotecan-based regimens in metastatic colorectal cancer
- PMID: 19859999
- PMCID: PMC2768885
- DOI: 10.3748/wjg.15.5058
UGT1A1 gene polymorphism: impact on toxicity and efficacy of irinotecan-based regimens in metastatic colorectal cancer
Abstract
Aim: To investigate the correlation between uridine diphosphate glucuronosyl transferase 1A1 (UGT1A1) gene polymorphisms and irinotecan-associated side effects and parameters of drug efficacy in patients with metastatic colorectal cancer (mCRC) receiving a low-dose weekly irinotecan chemotherapeutic regimen.
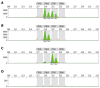
Methods: Genotypes were retrospectively evaluated by gene scan analysis on the ABI 310 sequencer of the TATAA box in the promoter region of the UGT1A1 gene in blood samples from 105 patients who had received 1st line irinotecan-based chemotherapy for mCRC.
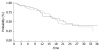
Results: The distribution of the genotypes was as follows: wild type genotype (WT) (6/6) 39.0%, heterozygous genotype (6/7) 49.5%, and homozygous genotype (7/7) 9.5%. The overall response rate (OR) was similar between patients carrying the (6/7, 7/7) or the WT genotype (6/6) (44.3% vs 43.2%, P = 0.75). Neither time to progression [(TTP) 8.1 vs 8.2 mo, P = 0.97] nor overall survival [(OS) 21.2 vs 18.9 mo, P = 0.73] differed significantly in patients who carried the (6/6) when compared to the (6/7, 7/7) genotype. No significant differences in toxicity were observed: Grade 3 and 4 delayed diarrhoea [(6/7, 7/7) vs (6/6); 13.0% vs 6.2%, P =0.08], treatment delays [(6/7, 7/7) vs (6/6); 25.1% vs 19.3%, P =0.24] or dose reductions [(6/7, 7/7) vs (6/6); 21.5% vs 27.2%, P =0.07].
Conclusion: This analysis demonstrates the non-significant influence of the UGT1A1 gene polymorphism on efficacy and rate of irinotecan-associated toxicity in mCRC patients receiving low-dose irinotecan based chemotherapy.
Figures

References
-
- Garcia-Carbonero R, Supko JG. Current perspectives on the clinical experience, pharmacology, and continued development of the camptothecins. Clin Cancer Res. 2002;8:641–661. - PubMed
-
- Ulukan H, Swaan PW. Camptothecins: a review of their chemotherapeutic potential. Drugs. 2002;62:2039–2057. - PubMed
-
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. - PubMed
-
- Folprecht G, Köhne CH. The role of new agents in the treatment of colorectal cancer. Oncology. 2004;66:1–17. - PubMed
-
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

