Hypothalamic FTO is associated with the regulation of energy intake not feeding reward
- PMID: 19860904
- PMCID: PMC2774323
- DOI: 10.1186/1471-2202-10-129
Hypothalamic FTO is associated with the regulation of energy intake not feeding reward
Abstract
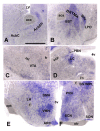
Background: Polymorphism in the FTO gene is strongly associated with obesity, but little is known about the molecular bases of this relationship. We investigated whether hypothalamic FTO is involved in energy-dependent overconsumption of food. We determined FTO mRNA levels in rodent models of short- and long-term intake of palatable fat or sugar, deprivation, diet-induced increase in body weight, baseline preference for fat versus sugar as well as in same-weight animals differing in the inherent propensity to eat calories especially upon availability of diverse diets, using quantitative PCR. FTO gene expression was also studied in organotypic hypothalamic cultures treated with anorexigenic amino acid, leucine. In situ hybridization (ISH) was utilized to study FTO signal in reward- and hunger-related sites, colocalization with anorexigenic oxytocin, and c-Fos immunoreactivity in FTO cells at initiation and termination of a meal.
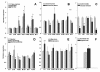
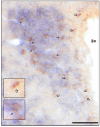
Results: Deprivation upregulated FTO mRNA, while leucine downregulated it. Consumption of palatable diets or macronutrient preference did not affect FTO expression. However, the propensity to ingest more energy without an effect on body weight was associated with lower FTO mRNA levels. We found that 4-fold higher number of FTO cells displayed c-Fos at meal termination as compared to initiation in the paraventricular and arcuate nuclei of re-fed mice. Moreover, ISH showed that FTO is present mainly in hunger-related sites and it shows a high degree of colocalization with anorexigenic oxytocin.
Conclusion: We conclude that FTO mRNA is present mainly in sites related to hunger/satiation control; changes in hypothalamic FTO expression are associated with cues related to energy intake rather than feeding reward. In line with that, neurons involved in feeding termination express FTO. Interestingly, baseline FTO expression appears linked not only with energy intake but also energy metabolism.
Figures

Similar articles
-
Fto immunoreactivity is widespread in the rodent brain and abundant in feeding-related sites, but the number of Fto-positive cells is not affected by changes in energy balance.Physiol Behav. 2011 May 3;103(2):248-53. doi: 10.1016/j.physbeh.2011.01.022. Epub 2011 Feb 2. Physiol Behav. 2011. PMID: 21295049
-
Fto colocalizes with a satiety mediator oxytocin in the brain and upregulates oxytocin gene expression.Biochem Biophys Res Commun. 2011 May 13;408(3):422-6. doi: 10.1016/j.bbrc.2011.04.037. Epub 2011 Apr 13. Biochem Biophys Res Commun. 2011. PMID: 21514276
-
FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting.PLoS One. 2011;6(11):e27968. doi: 10.1371/journal.pone.0027968. Epub 2011 Nov 30. PLoS One. 2011. PMID: 22140494 Free PMC article.
-
The 'Fat Mass and Obesity Related' (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance.Curr Obes Rep. 2015 Mar;4(1):73-91. doi: 10.1007/s13679-015-0143-1. Curr Obes Rep. 2015. PMID: 26627093 Review.
-
Oxytocin and Food Intake Control: Neural, Behavioral, and Signaling Mechanisms.Int J Mol Sci. 2021 Oct 8;22(19):10859. doi: 10.3390/ijms221910859. Int J Mol Sci. 2021. PMID: 34639199 Free PMC article. Review.
Cited by
-
Childhood obesity and the associated rise in cardiometabolic complications.Nat Metab. 2020 Mar;2(3):223-232. doi: 10.1038/s42255-020-0183-z. Epub 2020 Mar 16. Nat Metab. 2020. PMID: 32694781 Free PMC article. Review.
-
Gene-Environment Interplay in Child Eating Behaviors: What the Role of "Nature" Means for the Effects of "Nurture".Curr Nutr Rep. 2018 Dec;7(4):294-302. doi: 10.1007/s13668-018-0254-x. Curr Nutr Rep. 2018. PMID: 30374755 Free PMC article. Review.
-
N6-methyladenosine and Neurological Diseases.Mol Neurobiol. 2022 Mar;59(3):1925-1937. doi: 10.1007/s12035-022-02739-0. Epub 2022 Jan 15. Mol Neurobiol. 2022. PMID: 35032318 Review.
-
Preliminary findings on the influence of FTO rs9939609 and MC4R rs17782313 polymorphisms on resting energy expenditure, leptin and thyrotropin levels in obese non-morbid premenopausal women.J Physiol Biochem. 2014 Mar;70(1):255-62. doi: 10.1007/s13105-013-0300-5. Epub 2013 Dec 5. J Physiol Biochem. 2014. PMID: 24307561
-
Overexpression of Fto leads to increased food intake and results in obesity.Nat Genet. 2010 Dec;42(12):1086-92. doi: 10.1038/ng.713. Epub 2010 Nov 14. Nat Genet. 2010. PMID: 21076408 Free PMC article.
References
-
- Jacobsson JA, Klovins J, Kapa I, Danielsson P, Svensson V, Ridderstrale M, Gyllensten U, Marcus C, Fredriksson R, Schioth HB. Novel genetic variant in FTO influences insulin levels and insulin resistance in severely obese children and adolescents. Int J Obes (Lond) 2008;32:1730–5. doi: 10.1038/ijo.2008.168. - DOI - PubMed
-
- Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, Grallert H, Illig T, Wichmann HE, Rief W, et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

