Progranulin is expressed within motor neurons and promotes neuronal cell survival
- PMID: 19860916
- PMCID: PMC2779192
- DOI: 10.1186/1471-2202-10-130
Progranulin is expressed within motor neurons and promotes neuronal cell survival
Abstract
Background: Progranulin is a secreted high molecular weight growth factor bearing seven and one half copies of the cysteine-rich granulin-epithelin motif. While inappropriate over-expression of the progranulin gene has been associated with many cancers, haploinsufficiency leads to atrophy of the frontotemporal lobes and development of a form of dementia (frontotemporal lobar degeneration with ubiquitin positive inclusions, FTLD-U) associated with the formation of ubiquitinated inclusions. Recent reports indicate that progranulin has neurotrophic effects, which, if confirmed would make progranulin the only neuroprotective growth factor that has been associated genetically with a neurological disease in humans. Preliminary studies indicated high progranulin gene expression in spinal cord motor neurons. However, it is uncertain what the role of Progranulin is in normal or diseased motor neuron function. We have investigated progranulin gene expression and subcellular localization in cultured mouse embryonic motor neurons and examined the effect of progranulin over-expression and knockdown in the NSC-34 immortalized motor neuron cell line upon proliferation and survival.
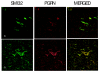
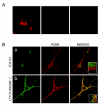
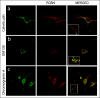
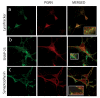
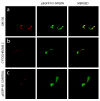
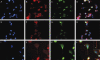
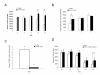
Results: In situ hybridisation and immunohistochemical techniques revealed that the progranulin gene is highly expressed by motor neurons within the mouse spinal cord and in primary cultures of dissociated mouse embryonic spinal cord-dorsal root ganglia. Confocal microscopy coupled to immunocytochemistry together with the use of a progranulin-green fluorescent protein fusion construct revealed progranulin to be located within compartments of the secretory pathway including the Golgi apparatus. Stable transfection of the human progranulin gene into the NSC-34 motor neuron cell line stimulates the appearance of dendritic structures and provides sufficient trophic stimulus to survive serum deprivation for long periods (up to two months). This is mediated at least in part through an anti-apoptotic mechanism. Control cells, while expressing basal levels of progranulin do not survive in serum free conditions. Knockdown of progranulin expression using shRNA technology further reduced cell survival.
Conclusion: Neurons are among the most long-lived cells in the body and are subject to low levels of toxic challenges throughout life. We have demonstrated that progranulin is abundantly expressed in motor neurons and is cytoprotective over prolonged periods when over-expressed in a neuronal cell line. This work highlights the importance of progranulin as neuroprotective growth factor and may represent a therapeutic target for neurodegenerative diseases including motor neuron disease.
Figures





References
-
- Plowman GD, Green JM, Neubauer MG, Buckley SD, McDonald VL, Todaro GJ, Shoyab M. The epithelin precursor encodes two proteins with opposing activities on epithelial cell growth. The Journal of Biological Chemistry. 1992;267:13073–13078. - PubMed
-
- Zhou J, Gao G, Crabb JW, Serrero G. Purification of an autocrine growth factor homologous with mouse epithelin presursor from a highly tumorigenic cell line. The Journal of Biological Chemistry. 1993;268:10863–10869. - PubMed
-
- Ong CH, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell derived growth factor, acrogranin) in proliferation and tumorigenesis. Histology and Histopathology. 2003;18:1275–1288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

