Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy
- PMID: 19861441
- PMCID: PMC2783253
- DOI: 10.1158/1078-0432.CCR-09-0371
Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy
Abstract
Purpose: Despite the strong association between malignant disease and thromboembolic disorders, the molecular and cellular basis of this relationship remains uncertain. We evaluated the hypothesis that tumor-derived tissue factor-bearing microparticles in plasma contribute to cancer-associated thrombosis.
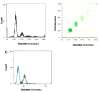
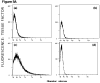
Experimental design: We developed impedance-based flow cytometry to detect, quantitate, and size microparticles in platelet-poor plasma. We evaluated the number of tissue factor-bearing microparticles in a cohort of cancer patients of different histologies (N = 96) and conducted a case-control study of 30 cancer patients diagnosed with an acute venous thromboembolic event (VTE) compared with 60 cancer patients of similar age, stage, sex, and diagnosis without known VTE, as well as 22 patients with an idiopathic VTE.
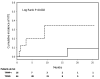
Results: Tissue factor-bearing microparticles were detected in patients with advanced malignancy, including two thirds of patients with pancreatic carcinoma. Elevated levels of tissue factor-bearing microparticles were associated VTE in cancer patients (adjusted odds ratio, 3.72; 95% confidence interval, 1.18-11.76; P = 0.01). In cancer patients without VTE, a retrospective analysis revealed a 1-year cumulative incidence of VTE of 34.8% in patients with tissue factor-bearing microparticles versus 0% in those without detectable tissue factor-bearing microparticles (Gray test P = 0.002).The median number of tissue factor-bearing microparticles in the cancer VTE cohort (7.1 x 10(4) microparticles/microL) was significantly greater than both the idiopathic VTE and cancer-no VTE groups (P = 0.002 and P = 0.03, respectively). Pancreatectomy in three patients eliminated or nearly eliminated these microparticles which coexpressed the epithelial tumor antigen, MUC-1.
Conclusion: We conclude that tumor-derived tissue factor-bearing microparticles are associated with VTE in cancer patients and may be central to the pathogenesis of cancer-associated thrombosis.
Figures




References
-
- Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983;62:14–31. - PubMed
-
- Donati MB. Cancer and thrombosis: from Phlegmasia alba dolens to transgenic mice. Thromb Haemost. 1995;74:278–81. - PubMed
-
- Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Semin Thromb Hemost. 1998;24:93–109. - PubMed
-
- Arkel YS. Thrombosis and cancer. Semin Oncol. 2000;27:362–74. - PubMed
-
- Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

