Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody
- PMID: 19861451
- PMCID: PMC2804258
- DOI: 10.1158/1078-0432.CCR-09-1066
Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody
Abstract
Purpose: Transforming growth factor-beta (TGF-beta) is an immunosuppressive cytokine, having direct suppressive activity against conventional CD4(+) and CD8(+)T cells and natural killer cells, thereby inhibiting tumor immunosurveillance. Here, we investigated possible synergy between anti-TGF-beta (1D11) and a peptide vaccine on induction of antitumor immunity, and the mechanisms accounting for synergistic efficacy.
Experimental design: The effect of combination treatment with a peptide vaccine and anti-TGF-beta was examined in a subcutaneous TC1 tumor model, as well as the mechanisms of protection induced by this treatment.
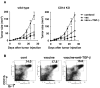
Results: Anti-TGF-beta significantly and synergistically improved vaccine efficacy as measured by reduction in primary tumor growth, although anti-TGF-beta alone had no impact. The number of tumor antigen-specific CTL with high functional avidity as measured by IFN-gamma production and lytic activity was significantly increased in vaccinated mice by TGF-beta neutralization. Although TGF-beta is known to play a critical role in CD4(+)Foxp3(+) Treg cells, Treg depletion/suppression by an anti-CD25 monoclonal antibody (PC61) before tumor challenge did not enhance vaccine efficacy, and adding anti-TGF-beta did not affect Treg numbers in lymph nodes or tumors or their function. Also, TGF-beta neutralization had no effect on interleukin-17-producing T cells, which are induced by TGF-beta and interleukin-6. Absence of type II NKT cells, which induce myeloid cells to produce TGF-beta, was not sufficient to eliminate all sources of suppressive TGF-beta. Finally, the synergistic protection induced by anti-TGF-beta vaccine augmentation was mediated by CD8(+) T cells since anti-CD8 treatment completely abrogated the effect.
Conclusions: These results suggest that TGF-beta blockade may be useful for enhancing cancer vaccine efficacy.
Figures
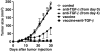


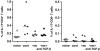
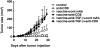
Comment in
-
Enhancing cancer vaccine efficacy via modulation of the tumor microenvironment.Clin Cancer Res. 2009 Nov 1;15(21):6476-8. doi: 10.1158/1078-0432.CCR-09-2256. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861446
Similar articles
-
Blockade of TGF-beta enhances tumor vaccine efficacy mediated by CD8(+) T cells.Int J Cancer. 2010 Apr 1;126(7):1666-74. doi: 10.1002/ijc.24961. Int J Cancer. 2010. PMID: 19830696 Free PMC article.
-
Systemic inhibition of transforming growth factor-beta in glioma-bearing mice improves the therapeutic efficacy of glioma-associated antigen peptide vaccines.Clin Cancer Res. 2009 Nov 1;15(21):6551-9. doi: 10.1158/1078-0432.CCR-09-1067. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861464 Free PMC article.
-
Effective induction of antitumor immunity by immunization with plasmid DNA encoding TRP-2 plus neutralization of TGF-beta.Cancer Immunol Immunother. 2005 May;54(5):446-52. doi: 10.1007/s00262-004-0619-4. Epub 2004 Nov 16. Cancer Immunol Immunother. 2005. PMID: 15750831 Free PMC article.
-
Efficacy Against Human Prostate Cancer by Prostate-specific Membrane Antigen-specific, Transforming Growth Factor-β Insensitive Genetically Targeted CD8+ T-cells Derived from Patients with Metastatic Castrate-resistant Disease.Eur Urol. 2018 May;73(5):648-652. doi: 10.1016/j.eururo.2017.12.008. Epub 2017 Dec 21. Eur Urol. 2018. PMID: 29275833 Free PMC article. Review.
-
TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression.Trends Immunol. 2010 Jun;31(6):220-7. doi: 10.1016/j.it.2010.04.002. Epub 2010 Jun 1. Trends Immunol. 2010. PMID: 20538542 Free PMC article. Review.
Cited by
-
Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation.Breast Cancer Res. 2016 Apr 1;18(1):38. doi: 10.1186/s13058-016-0698-0. Breast Cancer Res. 2016. PMID: 27036297 Free PMC article.
-
The immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the antigen-presenting function of CD40-activated B cells.J Exp Clin Cancer Res. 2012 May 16;31(1):47. doi: 10.1186/1756-9966-31-47. J Exp Clin Cancer Res. 2012. PMID: 22592077 Free PMC article.
-
Immunological effects of the TGFβ-blocking antibody GC1008 in malignant pleural mesothelioma patients.Oncoimmunology. 2013 Aug 1;2(8):e26218. doi: 10.4161/onci.26218. Epub 2013 Aug 27. Oncoimmunology. 2013. PMID: 24179709 Free PMC article.
-
Cancer Immunotherapy: Silencing Intracellular Negative Immune Regulators of Dendritic Cells.Cancers (Basel). 2019 Jan 17;11(1):108. doi: 10.3390/cancers11010108. Cancers (Basel). 2019. PMID: 30658461 Free PMC article. Review.
-
Japanese Kampo medicine ninjin'yoeito synergistically enhances tumor vaccine effects mediated by CD8+ T cells.Oncol Lett. 2017 May;13(5):3471-3478. doi: 10.3892/ol.2017.5937. Epub 2017 Mar 28. Oncol Lett. 2017. PMID: 28529575 Free PMC article.
References
-
- Terabe M, Matsui S, Park J-M, et al. Transforming Growth Factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block Cytotoxic T Lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. - PMC - PubMed
-
- Kobie JJ, Wu RS, Kurt RA, et al. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–4. - PubMed
-
- Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–8. - PubMed
-
- Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. - PubMed
-
- Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

