Increased sex chromosome expression and epigenetic abnormalities in spermatids from male mice with Y chromosome deletions
- PMID: 19861498
- PMCID: PMC2776507
- DOI: 10.1242/jcs.049916
Increased sex chromosome expression and epigenetic abnormalities in spermatids from male mice with Y chromosome deletions
Abstract
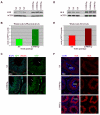
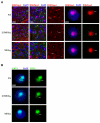
During male meiosis, the X and Y chromosomes are transcriptionally silenced, a process termed meiotic sex chromosome inactivation (MSCI). Recent studies have shown that the sex chromosomes remain substantially transcriptionally repressed after meiosis in round spermatids, but the mechanisms involved in this later repression are poorly understood. Mice with deletions of the Y chromosome long arm (MSYq-) have increased spermatid expression of multicopy X and Y genes, and so represent a model for studying post-meiotic sex chromosome repression. Here, we show that the increase in sex chromosome transcription in spermatids from MSYq- mice affects not only multicopy but also single-copy XY genes, as well as an X-linked reporter gene. This increase in transcription is accompanied by specific changes in the sex chromosome histone code, including almost complete loss of H4K8Ac and reduction of H3K9me3 and CBX1. Together, these data show that an MSYq gene regulates sex chromosome gene expression as well as chromatin remodelling in spermatids.
Figures


Similar articles
-
Deletions on mouse Yq lead to upregulation of multiple X- and Y-linked transcripts in spermatids.Hum Mol Genet. 2005 Sep 15;14(18):2705-15. doi: 10.1093/hmg/ddi304. Epub 2005 Aug 8. Hum Mol Genet. 2005. PMID: 16087683
-
Expression and epigenomic landscape of the sex chromosomes in mouse post-meiotic male germ cells.Epigenetics Chromatin. 2016 Oct 27;9:47. doi: 10.1186/s13072-016-0099-8. eCollection 2016. Epigenetics Chromatin. 2016. PMID: 27795737 Free PMC article.
-
SSTY proteins co-localize with the post-meiotic sex chromatin and interact with regulators of its expression.FEBS J. 2014 Mar;281(6):1571-84. doi: 10.1111/febs.12724. Epub 2014 Feb 13. FEBS J. 2014. PMID: 24456183 Free PMC article.
-
Epigenetic mechanisms of gene regulation during mammalian spermatogenesis.Epigenetics. 2008 Jan-Feb;3(1):21-8. doi: 10.4161/epi.3.1.5555. Epigenetics. 2008. PMID: 18416029 Review.
-
Histone crotonylation specifically marks the haploid male germ cell gene expression program: post-meiotic male-specific gene expression.Bioessays. 2012 Mar;34(3):187-93. doi: 10.1002/bies.201100141. Epub 2011 Dec 15. Bioessays. 2012. PMID: 22170506 Review.
Cited by
-
Effects of the Sex Chromosome Complement, XX, XO, or XY, on the Transcriptome and Development of Mouse Oocytes During Follicular Growth.Front Genet. 2021 Dec 20;12:792604. doi: 10.3389/fgene.2021.792604. eCollection 2021. Front Genet. 2021. PMID: 34987552 Free PMC article.
-
Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm.Genes (Basel). 2019 Feb 12;10(2):133. doi: 10.3390/genes10020133. Genes (Basel). 2019. PMID: 30759861 Free PMC article.
-
The contribution of the Y chromosome to hybrid male sterility in house mice.Genetics. 2012 Aug;191(4):1271-81. doi: 10.1534/genetics.112.141804. Epub 2012 May 17. Genetics. 2012. PMID: 22595240 Free PMC article.
-
Deficiency in mouse Y chromosome long arm gene complement is associated with sperm DNA damage.Genome Biol. 2010;11(6):R66. doi: 10.1186/gb-2010-11-6-r66. Epub 2010 Jun 23. Genome Biol. 2010. PMID: 20573212 Free PMC article.
-
Sex chromosome inactivation in germ cells: emerging roles of DNA damage response pathways.Cell Mol Life Sci. 2012 Aug;69(15):2559-72. doi: 10.1007/s00018-012-0941-5. Epub 2012 Mar 2. Cell Mol Life Sci. 2012. PMID: 22382926 Free PMC article. Review.
References
-
- Aravin, A. A., Naumova, N. M., Tulin, A. V., Vagin, V. V., Rozovsky, Y. M. and Gvozdev, V. A. (2001). Double-stranded RNA-mediated silencing of the genomic tandem repeats and transposable elements in the D. melanogaster germline. Curr. Biol. 11, 1017-1027. - PubMed
-
- Baarends, W. M., Hoogerbrugge, J. W., Roest, H. P., Oooms, M., Vreeburg, J., Hoeijmakers, J. H. J. and Grootegoed, J. A. (1999). Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev. Biol. 207, 322-333. - PubMed
-
- Baarends, W. M., Wassenaar, E., Hoogerbrugge, J. W., Schoenmakers, S., Sun, Z. W. and Grootegoed, J. A. (2007). Increased phosphorylation and dimethylation of XY body histones in the Hr6b-knockout mouse is associated with de-repression of the X chromosome. J. Cell Sci. 120, 1841-1851. - PubMed
-
- Burgoyne, P. S., Mahadevaiah, S. K., Sutcliffe, M. J. and Palmer, S. J. (1992). Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell 71, 391-398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

