Proteomic Analysis of Anti-inflammatory Effects of a Kampo (Japanese Herbal) Medicine "Shoseiryuto (Xiao-Qing-Long-Tang)" on Airway Inflammation in a Mouse Model
- PMID: 19861507
- PMCID: PMC3136714
- DOI: 10.1093/ecam/nep151
Proteomic Analysis of Anti-inflammatory Effects of a Kampo (Japanese Herbal) Medicine "Shoseiryuto (Xiao-Qing-Long-Tang)" on Airway Inflammation in a Mouse Model
Abstract
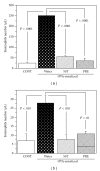
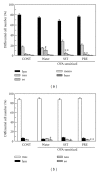
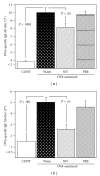
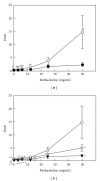
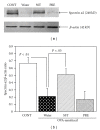
Effects of a Kampo (Japanese herbal) medicine "shoseiryuto (SST, xiao-qing-long-tang in Chinese)", which has been used for the treatment of allergic bronchial asthma clinically, were examined on ovalbumin (OVA)-sensitized allergic airway inflammation model (i.e., bronchial asthma) in a mouse. When SST was orally administered at 0.5 g kg(-1) day(-1) from day 1 to 6 after OVA inhalation, SST reduced the inflammation in lung tissue, the number of eosinophils and the OVA-specific immunoglobulin E (IgE) antibody titer in bronchoalveolar lavage (BAL) fluids at 7 days after the OVA inhalation. SST also reduced the airway hyperreactivity at 6 days after the OVA inhalation. Proteomic analysis with the agarose two-dimensional electrophoresis showed that the expression of spectrin α2 was reduced in the lung tissue of OVA-sensitized mice and SST recovered the expression. Western blot and immunohistochemical analyses of lung tissue also confirmed this result. When prednisolone was orally administered at 3 mg kg(-1) day(-1) from day 1 to 6 after OVA inhalation, the inflammation in lung tissue, the number of eosinophils in BAL fluids and airway hyperreactivity were reduced in the OVA-sensitized mice. However, prednisolone did not reduce the OVA-specific IgE antibody titer in BAL fluids and did not recover the expression of spectrin α2 in lung tissue. These results suggest that at least a part of action mechanism of SST against OVA-sensitized allergic airway inflammation in a mouse model is different from that of prednisolone.
Figures



Similar articles
-
Anti-allergic activity of a Kampo (Japanese herbal) medicine "Sho-seiryu-to (Xiao-Qing-Long-Tang)" on airway inflammation in a mouse model.Int Immunopharmacol. 2004 Oct;4(10-11):1353-65. doi: 10.1016/j.intimp.2004.05.021. Int Immunopharmacol. 2004. PMID: 15313433
-
Enhancement of anti-allergic effects mediated by the Kampo medicine Shoseiryuto (Xiao-Qing-Long-Tang in Chinese) with lysed Enterococcus faecalis FK-23 in mice.Asian Pac J Allergy Immunol. 2010 Mar;28(1):59-66. Asian Pac J Allergy Immunol. 2010. PMID: 20527518
-
4-1 BB stimulation inhibits allergen-specific immunoglobulin E production and airway hyper-reactivity but partially suppresses bronchial eosinophilic inflammation in a mouse asthma model.Clin Exp Allergy. 2006 Mar;36(3):377-85. doi: 10.1111/j.1365-2222.2006.02445.x. Clin Exp Allergy. 2006. PMID: 16499650
-
The involvement of type 1a angiotensin II receptors in the regulation of airway inflammation in a murine model of allergic asthma.Clin Exp Allergy. 2007 Nov;37(11):1720-7. doi: 10.1111/j.1365-2222.2007.02815.x. Epub 2007 Sep 17. Clin Exp Allergy. 2007. PMID: 17877756
-
Continued inhalation of lidocaine suppresses antigen-induced airway hyperreactivity and airway inflammation in ovalbumin-sensitized guinea pigs.Int Immunopharmacol. 2008 May;8(5):725-31. doi: 10.1016/j.intimp.2008.01.021. Epub 2008 Feb 20. Int Immunopharmacol. 2008. PMID: 18387515
Cited by
-
The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies.Allergy Asthma Clin Immunol. 2015 Mar 16;11(1):11. doi: 10.1186/s13223-015-0074-0. eCollection 2015. Allergy Asthma Clin Immunol. 2015. PMID: 25897313 Free PMC article.
-
Soluble toll-like receptor 4 reversed attenuating effect of Chinese herbal Xiao-Qing-Long-Tang on allergen induced nerve growth factor and thymic stromal lymphopoietin.Exp Ther Med. 2013 Nov;6(5):1199-1207. doi: 10.3892/etm.2013.1294. Epub 2013 Sep 13. Exp Ther Med. 2013. PMID: 24223644 Free PMC article.
-
Traditional Chinese medicine classic herbal formula Xiaoqinglong decoction for acute exacerbation of chronic obstructive pulmonary disease: A systematic review protocol.Medicine (Baltimore). 2018 Dec;97(52):e13761. doi: 10.1097/MD.0000000000013761. Medicine (Baltimore). 2018. PMID: 30593152 Free PMC article.
-
Alleviative Effects of a Kampo (a Japanese Herbal) Medicine "Maoto (Ma-Huang-Tang)" on the Early Phase of Influenza Virus Infection and Its Possible Mode of Action.Evid Based Complement Alternat Med. 2014;2014:187036. doi: 10.1155/2014/187036. Epub 2014 Mar 20. Evid Based Complement Alternat Med. 2014. PMID: 24778699 Free PMC article.
-
Identifying the Chinese Herbal Medicine Network and Core Formula for Allergic Rhinitis on a Real-World Database.Evid Based Complement Alternat Med. 2020 Nov 5;2020:5979708. doi: 10.1155/2020/5979708. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33204289 Free PMC article.
References
-
- Fanta CH. Asthma. New England Journal of Medicine. 2009;360(10):1002–1014. - PubMed
-
- Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased T(H2)-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. Journal of Allergy and Clinical Immunology. 1993;92(2):313–324. - PubMed
-
- Renz H, Smith HR, Henson JE, Ray BS, Irvin CG, Gelfand EW. Aerosolized antigen exposure without adjuvant causes increased IgE production and increased airway responsiveness in the mouse. Journal of Allergy and Clinical Immunology. 1992;89(6):1127–1138. - PubMed
-
- Lenfant C, Khaltaev N, editors. Global Initiative for Asthma: Global Strategy for Asthma Management and Prevention NHLBI/WHO Workshop Report. Bethesda, Md, USA: National Institute of Health, National Heart, Lung, and Blood Institute; 1995. (NIH Publication, no. 95-3659).
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

