Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate
- PMID: 19861682
- PMCID: PMC2978503
- DOI: 10.1182/blood-2009-04-219253
Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate
Abstract
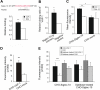
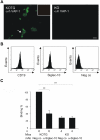
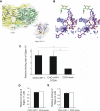
Leukocytes migrate from the blood into areas of inflammation by interacting with various adhesion molecules on endothelial cells. Vascular adhesion protein-1 (VAP-1) is a glycoprotein expressed on inflamed endothelium where it plays a dual role: it is both an enzyme that oxidizes primary amines and an adhesin that is involved in leukocyte trafficking to sites of inflammation. Although VAP-1 was identified more than 15 years ago, the counterreceptor(s) for VAP-1 on leukocytes has remained unknown. Here we have identified Siglec-10 as a leukocyte ligand for VAP-1 using phage display screenings. The binding between Siglec-10 and VAP-1 was verified by different adhesion assays, and this interaction was also consistent with molecular modeling. Moreover, the interaction between Siglec-10 and VAP-1 led to increased hydrogen peroxide production, indicating that Siglec-10 serves as a substrate for VAP-1. Thus, the Siglec-10-VAP-1 interaction seems to mediate lymphocyte adhesion to endothelium and has the potential to modify the inflammatory microenvironment via the enzymatic end products.
Figures

References
-
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24(6):327–334. - PubMed
-
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3(11):867–878. - PubMed
-
- Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257(5075):1407–1409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

