Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs
- PMID: 19861690
- PMCID: PMC2770878
- DOI: 10.1126/scisignal.2000442
Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs
Abstract
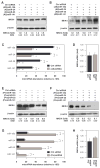
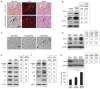
MKK4 (mitogen-activated protein kinase kinase 4) is a pivotal upstream activator of c-Jun N-terminal kinase and p38. Here, we report that the abundance of MKK4 increases in senescent human diploid fibroblasts through enhanced translation. We identified four microRNAs (miR-15b, miR-24, miR-25, and miR-141) that target the MKK4 messenger RNA (mRNA); the abundance of these microRNAs decreased during replicative senescence. Individually modulating the amount of each microRNA did not modify MKK4 abundance, but their concomitant overexpression decreased and their joint reduction increased MKK4 abundance. Reporter analyses indicated that these microRNAs acted through the MKK4 5' and 3' untranslated regions. Elevated MKK4 abundance inhibited cell proliferation and increased the phosphorylation and activity of p38 and PRAK (p38-regulated/activated protein kinase). Thus, multiple microRNAs acting on a single target, the MKK4 mRNA, collectively influence MKK4 abundance during replicative senescence.
Figures


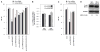

Comment in
-
Comment on "Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs".Sci Signal. 2010 Jul 20;3(131):lc1; author reply lc2. doi: 10.1126/scisignal.3131lc1. Sci Signal. 2010. PMID: 20647589
References
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. - PubMed
-
- Cristofalo VJ, Lorenzini A, Allen RG, Torres C, Tresini M. Replicative senescence: a critical review. Mech Ageing Dev. 2004;125:827–848. - PubMed
-
- Smith JR, Pereira-Smith OM. Replicative senescence: implications for in vivo aging and tumor suppression. Science. 1996;273:63–67. - PubMed
-
- Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

