Systematic evaluation of AAV vectors for liver directed gene transfer in murine models
- PMID: 19861950
- PMCID: PMC2839210
- DOI: 10.1038/mt.2009.246
Systematic evaluation of AAV vectors for liver directed gene transfer in murine models
Abstract
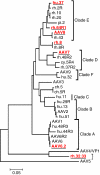
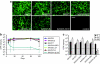
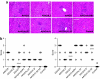
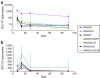
Vectors based on adeno-associated viruses (AAVs) are being evaluated for use in liver-directed gene therapy. Candidates have been preselected on the basis of capsid structure that plays an important role in determining performance profiles. We describe a comprehensive and statistically powered set of mouse studies designed to compare the performance of vectors based on seven novel AAV capsids. The key criteria used to select candidates for successful gene therapy are high level and stable transgene expression in the absence of toxicity. Based on these criteria, the best performing vectors, AAV8, AAVhu.37, and AAVrh.8, will be further evaluated in nonhuman primates (NHPs).
Figures


References
-
- Nguyen TH, Mainot S, Lainas P, Groyer-Picard MT, Franco D, Dagher I, et al. Ex vivo liver-directed gene therapy for the treatment of metabolic diseases: advances in hepatocyte transplantation and retroviral vectors. Curr Gene Ther. 2009;9:136–149. - PubMed
-
- Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. - PubMed
-
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. - PubMed
-
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. - PubMed
-
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

