Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy
- PMID: 19861951
- PMCID: PMC2839224
- DOI: 10.1038/mt.2009.247
Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy
Abstract
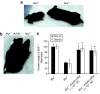
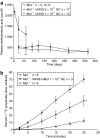
Methylmalonic acidemia (MMA) is an organic acidemia caused by deficient activity of the mitochondrial enzyme methylmalonyl-CoA mutase (MUT). This disorder is associated with lethal metabolic instability and carries a poor prognosis for long-term survival. A murine model of MMA that replicates a severe clinical phenotype was used to examine the efficacy of recombinant adeno-associated virus (rAAV) serotype 8 gene therapy as a treatment for MMA. Lifespan extension, body weight, circulating metabolites, transgene expression, and whole animal propionate oxidation were examined as outcome parameters after gene therapy. One-hundred percent of the untreated Mut(-/-) mice (n = 58) died by day of life (DOL) 72, whereas >95% of the adeno-associated virus-treated Mut(-/-) mice (n = 27) have survived for > or = 1 year. Despite a gradual loss of transgene expression and elevated circulating metabolites in the treated Mut(-/-) mice, the animals are indistinguishable from unaffected control littermates in size and activity levels. These experiments provide the first definitive evidence that gene therapy will have clinical utility in the treatment of MMA and support the development of gene therapy for other organic acidemias.
Figures



References
-
- Fenton WA, Gravel RA., and , Rosenblatt DS.2001Disorders of propionate and methylmalonate metabolismIn: Scriver, CR, Sly, WS, Childs, B, Beaudet AL, Valle, D, Kinzler, KW et al. (eds). The Metabolic and Molecular Bases of Inherited Disease, 8th edn. McGraw-Hill: New York. pp. 2165–2192.
-
- Matsui SM, Mahoney MJ., and , Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308:857–861. - PubMed
-
- van der Meer SB, Poggi F, Spada M, Bonnefont JP, Ogier H, Hubert P, et al. 1994Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia J Pediatr 1256 Pt 1): 903–908. - PubMed
-
- de Baulny HO, Benoist JF, Rigal O, Touati G, Rabier D., and , Saudubray JM. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28:415–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

