Structural basis of the aberrant receptor binding properties of hagfish and lamprey insulins
- PMID: 19863112
- PMCID: PMC2781304
- DOI: 10.1021/bi901269j
Structural basis of the aberrant receptor binding properties of hagfish and lamprey insulins
Abstract
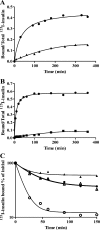
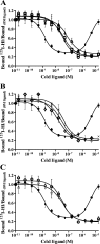
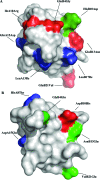
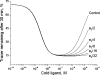
The insulin from the Atlantic hagfish (Myxine glutinosa) has been one of the most studied insulins from both a structural and a biological viewpoint; however, some aspects of its biology remain controversial, and there has been no satisfying structural explanation for its low biological potency. We have re-examined the receptor binding kinetics, as well as the metabolic and mitogenic properties, of this phylogenetically ancient insulin, as well as that from another extant representative of the ancient chordates, the river lamprey (Lampetra fluviatilis). Both insulins share unusual binding kinetics and biological properties with insulin analogues that have single mutations at residues that contribute to the hexamerization surface. We propose and demonstrate by reciprocal amino acid substitutions between hagfish and human insulins that the reduced biological activity of hagfish insulin results from unfavorable substitutions, namely, A10 (Ile to Arg), B4 (Glu to Gly), B13 (Glu to Asn), and B21 (Glu to Val). We likewise suggest that the altered biological activity of lamprey insulin may reflect substitutions at A10 (Ile to Lys), B4 (Glu to Thr), and B17 (Leu to Val). The substitution of Asp at residue B10 in hagfish insulin and of His at residue A8 in both hagfish and lamprey insulins may help compensate for unfavorable changes in other regions of the molecules. The data support the concept that the set of unusual properties of insulins bearing certain mutations in the hexamerization surface may reflect those of the insulins evolutionarily closer to the ancestral insulin gene product.
Figures




References
-
- De Meyts P. (2004) Insulin and its receptor: Structure, function and evolution. BioEssays 26, 1351–1362. - PubMed
-
- Hernandez-Sanchez C.; Mansilla A.; de Pablo F.; Zardoya R. (2008) Evolution of the insulin receptor family and receptor isoform expression in vertebrates. Mol. Biol. Evol. 25, 1043–1053. - PubMed
-
- Wilkinson T. N.; Bathgate R. A. (2007) The evolution of the relaxin peptide family and their receptors. Adv. Exp. Med. Biol. 612, 1–13. - PubMed
-
- Bathgate R. A.; Ivell R.; Sanborn B. M.; Sherwood O. D.; Summers R. J. (2005) Receptors for relaxin family peptides. Ann. N.Y. Acad. Sci. 1041, 61–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

