Emerging evidence of a link between the polycystins and the mTOR pathways
- PMID: 19863783
- PMCID: PMC2781793
- DOI: 10.1186/1755-8417-2-6
Emerging evidence of a link between the polycystins and the mTOR pathways
Abstract
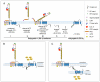
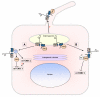
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disease characterized by the formation of renal cysts. This disease can be caused by mutations in two genes, PKD1 and PKD2, which encode polycystin-1 (PC-1) and -2 (PC-2), respectively.PC-1 is a large plasma membrane receptor involved in the regulation of several biological functions and signaling pathways, and PC-2 is a calcium channel of the TRP family. The two proteins associate in a complex to prevent cyst formation, but the precise mechanism(s) involved remain largely unknown.This review will focus on recent advances in our understanding of the functions of polycystins and their role in signal transduction.Increased activity of the mammalian target of rapamycin (mTOR) kinase has been observed in cysts found in ADPKD tissues. Rapamycin has been shown to have beneficial effects in rodent models of polycystic kidney disease, prompting the initiation of pilot clinical trials with human patients. Furthermore, a direct role for PC-1 in the regulation of cell growth (size) via mTOR has recently been demonstrated.Major advancements in the study of mTOR biology have highlighted that this kinase exists in association with two different complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). The mTORC1 complex regulates cell growth (size), proliferation, translation and autophagy, and mTORC2 regulates the actin cytoskeleton and apoptosis. Interestingly, mTORC2 has been shown to contain the kinase responsible for the phosphorylation of Akt at Serine 473. Previous studies have shown that PC-1 controls the PI 3-kinase/Akt cascade to regulate apoptosis and the actin cytoskeleton, suggesting that this receptor might regulate mTOR at several levels.This review aims to discuss three different, inter-related themes emerging from the literature: (i) studies performed in our and other laboratories collectively suggest that PC-1 might be able to differentially regulate the two mTOR complexes; (ii) several studies point to genetic and functional cross-talk between the PKD and TSC genes, although the molecular details remain obscure; and (iii) studies performed in mammals and in the unicellular algae Chlamidomonas Reinhardtii might highlight a link between cilia, regulation of cell size and regulation of the cell cycle.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

