Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling
- PMID: 19864425
- PMCID: PMC2804154
- DOI: 10.1074/jbc.M109.040428
Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling
Abstract
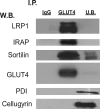
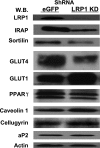
Insulin stimulates the translocation of intracellular GLUT4 to the plasma membrane where it functions in adipose and muscle tissue to clear glucose from circulation. The pathway and regulation of GLUT4 trafficking are complicated and incompletely understood and are likely to be contingent upon the various proteins other than GLUT4 that comprise and interact with GLUT4-containing vesicles. Moreover, not all GLUT4 intracellular pools are insulin-responsive as some represent precursor compartments, thus posing a biochemical challenge to the purification and characterization of their content. To address these issues, we immunodepleted precursor GLUT4-rich vesicles and then immunopurified GLUT4 storage vesicle (GSVs) from primary rat adipocytes and subjected them to semi-quantitative and quantitative proteomic analysis. The purified vesicles translocate to the cell surface almost completely in response to insulin, the expected behavior for bona fide GSVs. In total, over 100 proteins were identified, about 50 of which are novel in this experimental context. LRP1 (low density lipoprotein receptor-related protein 1) was identified as a major constituent of GSVs, and we show it interacts with the lumenal domains of GLUT4 and other GSV constituents. Its cytoplasmic tail interacts with the insulin-signaling pathway target, AS160 (Akt substrate of 160 kDa). Depletion of LRP1 from 3T3-L1 adipocytes reduces GLUT4 expression and correspondingly results in decreased insulin-stimulated 2-[(3)H]deoxyglucose uptake. Furthermore, adipose-specific LRP1 knock-out mice also exhibit decreased GLUT4 expression. These findings suggest LRP1 is an important component of GSVs, and its expression is needed for the formation of fully functional GSVs.
Figures








References
-
- Huang S., Czech M. P. (2007) Cell Metab. 5, 237–252 - PubMed
-
- Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., Pilch P. F. (1989) J. Biol. Chem. 264, 12358–12363 - PubMed
-
- James D. E., Brown R., Navarro J., Pilch P. F. (1988) Nature 333, 183–185 - PubMed
-
- Cushman S. W., Wardzala L. J. (1980) J. Biol. Chem. 255, 4758–4762 - PubMed
-
- Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., Cushman S. W. (1990) J. Biol. Chem. 265, 18172–18179 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

