Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology
- PMID: 19864480
- PMCID: PMC2812290
- DOI: 10.1128/JCM.01463-09
Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology
Abstract
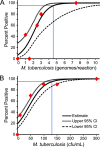
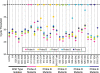
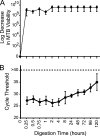
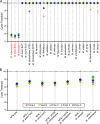
Current nucleic acid amplification methods to detect Mycobacterium tuberculosis are complex, labor-intensive, and technically challenging. We developed and performed the first analysis of the Cepheid Gene Xpert System's MTB/RIF assay, an integrated hands-free sputum-processing and real-time PCR system with rapid on-demand, near-patient technology, to simultaneously detect M. tuberculosis and rifampin resistance. Analytic tests of M. tuberculosis DNA demonstrated a limit of detection (LOD) of 4.5 genomes per reaction. Studies using sputum spiked with known numbers of M. tuberculosis CFU predicted a clinical LOD of 131 CFU/ml. Killing studies showed that the assay's buffer decreased M. tuberculosis viability by at least 8 logs, substantially reducing biohazards. Tests of 23 different commonly occurring rifampin resistance mutations demonstrated that all 23 (100%) would be identified as rifampin resistant. An analysis of 20 nontuberculosis mycobacteria species confirmed high assay specificity. A small clinical validation study of 107 clinical sputum samples from suspected tuberculosis cases in Vietnam detected 29/29 (100%) smear-positive culture-positive cases and 33/39 (84.6%) or 38/53 (71.7%) smear-negative culture-positive cases, as determined by growth on solid medium or on both solid and liquid media, respectively. M. tuberculosis was not detected in 25/25 (100%) of the culture-negative samples. A study of 64 smear-positive culture-positive sputa from retreatment tuberculosis cases in Uganda detected 63/64 (98.4%) culture-positive cases and 9/9 (100%) cases of rifampin resistance. Rifampin resistance was excluded in 54/55 (98.2%) susceptible cases. Specificity rose to 100% after correcting for a conventional susceptibility test error. In conclusion, this highly sensitive and simple-to-use system can detect M. tuberculosis directly from sputum in less than 2 h.
Figures
References
-
- Association for the Advancement of Medical Instrumentation. 2003. Sterilization of health care products—requirements for products labeled “sterile.” Standard ANSI/AAMI ST67. Association for the Advancement of Medical Instrumentation, Arlington, VA.
-
- Barnard, M., H. Albert, G. Coetzee, R. O'Brien, and M. E. Bosman. 2008. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am. J. Respir. Crit. Care Med. 177:787-792. - PubMed
-
- Campos, M., A. Quartin, E. Mendes, A. Abreu, S. Gurevich, L. Echarte, T. Ferreira, T. Cleary, E. Hollender, and D. Ashkin. 2008. Feasibility of shortening respiratory isolation with a single sputum nucleic acid amplification test. Am. J. Respir. Crit. Care Med. 178:300-305. - PubMed
-
- Caviedes, L., T. S. Lee, R. H. Gilman, P. Sheen, E. Spellman, E. H. Lee, D. E. Berg, S. Montenegro-James, and the Tuberculosis Working Group in Peru. 2000. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J. Clin. Microbiol. 38:1203-1208. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

