Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord
- PMID: 19864556
- PMCID: PMC2788152
- DOI: 10.1523/JNEUROSCI.3257-09.2009
Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord
Abstract
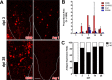
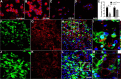
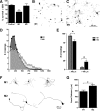
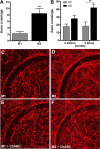
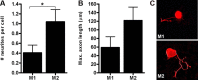
Macrophages dominate sites of CNS injury in which they promote both injury and repair. These divergent effects may be caused by distinct macrophage subsets, i.e., "classically activated" proinflammatory (M1) or "alternatively activated" anti-inflammatory (M2) cells. Here, we show that an M1 macrophage response is rapidly induced and then maintained at sites of traumatic spinal cord injury and that this response overwhelms a comparatively smaller and transient M2 macrophage response. The high M1/M2 macrophage ratio has significant implications for CNS repair. Indeed, we present novel data showing that only M1 macrophages are neurotoxic and M2 macrophages promote a regenerative growth response in adult sensory axons, even in the context of inhibitory substrates that dominate sites of CNS injury (e.g., proteoglycans and myelin). Together, these data suggest that polarizing the differentiation of resident microglia and infiltrating blood monocytes toward an M2 or "alternatively" activated macrophage phenotype could promote CNS repair while limiting secondary inflammatory-mediated injury.
Figures


References
-
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. - PubMed
-
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16:851–863. - PubMed
-
- Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60:263–273. - PubMed
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
