Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain
- PMID: 19864566
- PMCID: PMC2787190
- DOI: 10.1523/JNEUROSCI.3362-09.2009
Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain
Abstract
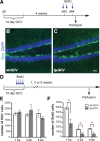
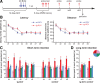
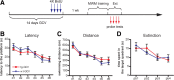
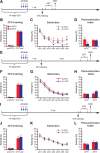
Adult-born dentate granule cells (DGCs) contribute to learning and memory, yet it remains unknown when adult-born DGCs become involved in the cognitive processes. During neurogenesis, immature DGCs display distinctive physiological characteristics while undergoing morphological maturation before final integration into the neural circuits. The survival and activity of the adult-born DGCs can be influenced by the experience of the animal during a critical period when newborn DGCs are still immature. To assess the temporal importance of adult neurogenesis, we developed a transgenic mouse model that allowed us to transiently reduce the numbers of adult-born DGCs in a temporally regulatable manner. We found that mice with a reduced population of adult-born DGCs at the immature stage were deficient in forming robust, long-term spatial memory and displayed impaired performance in extinction tasks. These results suggest that immature DGCs that undergo maturation make important contributions to learning and memory.
Figures



References
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Bath KG, Mandairon N, Jing D, Rajagopal R, Kapoor R, Chen ZY, Khan T, Proenca CC, Kraemer R, Cleland TA, Hempstead BL, Chao MV, Lee FS. Variant brain-derived neurotrophic factor (Val66Met) alters adult olfactory bulb neurogenesis and spontaneous olfactory discrimination. J Neurosci. 2008;28:2383–2393. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
